HSC Chemistry Syllabus Notes
Module 5 / Inquiry Question 1
Overview of Week 1’s Inquiry Question
Learning Objective #1 – Static & Dynamic Equilibrium
Learning Objective #2 – Experiments on reversibility of chemical reactions
Learning Objective #3 – Static and dynamic equilibrium models
Learning Objective #4 – Analyse differences for open and closed systems
Learning Objective #5 – Non-equilibrium systems’ Entropy & Enthalpy
Learning Objective #6 – Collision Theory & Reaction Rate in equilibrium reactions
HSC Chemistry Syllabus Lecture Video – Static & Dynamic Equilibrium
Week 1 Homework Set (Essential for Band 5)
Curveball Questions (Moving from Band 5 to Band 6!)
Week 1 Extension Questions (Extra HSC-style questions)
Solutions to Week 1 Questions
Overview of Week 1's Inquiry Question
Welcome to Week 1 of your Year 12 HSC Chemistry Syllabus Notes!
From this point onwards, I will assume that you have read all the materials from the Year 11 HSC Chemistry Syllabus Notes. If not, please do so now.
Getting the fundamentals of chemistry will set you up for success.
You will spend less time and effort revising for your chemistry exams.
Before we hop on the materialistic train and start digging into the course material, please allow me a minute to walk you through what you should keep in mind as the ‘major highlights’ of this week’s material.
The inquiry question for this week deals with the chemical reactions that do not proceed to completion.
In the Year 11 HSC Chemistry Syllabus Notes, most of the chemical reactions that you wrote are those that proceeded to completion.
For example, your teacher may have burned a thin strip of magnesium where you saw a bright white glow, resulting in the magnesium metal turning into what you see as white solid (magnesium oxide, MgO).
However, there are chemical reactions that DO NOT proceed to completion and in fact the products can react with each other and reform the initial reactants! Wow? Yes.
These will be chemical reactions, which we call equilibrium reactions, that do not proceed to completion as the reactants and products are constantly reacting with themselves, converting to between each other.
Within the category of equilibrium reactions, there is static and dynamic equilibrium systems.
A system is an environment where there is one or more chemical reactions taking place.
These equilibrium systems may be opened or closed.
Open systems are subjected to influence of environmental factors such as temperature, pressure, foreign substances entering the system, thus, altering the chemical reaction equation.
Closed systems are not subjected to the influence of environmental factors, however, we can manipulate the environmental conditions of the closed system using various methods and technologies.
Every chemical system, including equilibrium systems, has its own entropy and enthalpy.
Although we have talked about entropy and enthalpy in preliminary HSC Chemistry, we will explore what these two terms mean again here and assess the impact these concepts have on a chemical reaction in dynamic equilibrium.
At the final section of this week’s notes, we will cover the effects of the collision theory and reaction rates on equilibrium reactions.
Learning Objective #1 – Distinguish between static & dynamic equilibrium
What is equilibrium?
It is important to note that the word ‘equilibrium’ always come with the symbol, ⇌
Whenever you see this symbol, ⇌, you should automatically note to yourself that anything on the left of the chemical equation are being converted into whatever that is on the right of the equation and vice versa.
For example, suppose we have an equilibrium reaction (denoted by ⇌)
Compound A + Compound B ⇌ Compound C + Compound D
You can interpret the above equilibrium reaction as Compound A is reacting with Compound B to produce Compounds C and D.
However, at the SAME TIME, when the reaction has reached or is at equilibrium, Compounds C and D are also reacting to produce Compounds A and B at the SAME RATE.
This means that the rate at which A and B are being consumed is equal to the rate at which A and B are being formed (from C and D reacting together).
This also means that at the state of chemical equilibrium, the rate of the forward and reverse reaction occur at the same rate.
Rate of forward reaction = Rate of reverse reaction.
This is always true for all the types of equilibrium that we will explore later on , so remember it.
Thus, the rate of change and reversal of the change are the same!
You may ask, wait.. does this mean that if you observe a reaction that is AT chemical equilibrium (visually from your naked eye), you will not see any changes in visual appearance of the system? YEP!
However, at a molecular level, there will definitely be movement of compounds, i.e. chemical bonds breaking and forming.
It is also important to note that the CHANGE IN CONCENTRATION of Compound A, B, C and D in the equilibrium reaction are CONSTANT.
Using the same generic equilibrium reaction we used above, if one mole of compound A is reacted with one mole of compound B to form products (compounds C and D), then the products will react together to form one mole of A again. This means that the concentration of A in the equilibrium system will always be constant.
That is, at equilibrium, compound A will decrease and increase by the same amount that is consistent with its mole ratio at any given time. This is true for all other reacting species (reactants and products) that is involved in the equilibrium reaction, i.e. compound B, C and D.
Constant change in concentration DOES NOT necessarily mean that there is an equal change in concentration for all species (reactant and products) participating in the equilibrium reaction. This is because the increase/decrease in concentration amount depends on the molar ratio of the participating species!
For example, say there is the following equilibrium reaction:
2A + B ⇌ 4C + D
Here, two moles of substance A is required to react with one mole of substance B to form four moles C and one mole of D.
Vice versa, four moles of substance C is required to react with one mole of substance D to form 2 moles of substance A and one mole of substance B.
Hence, the change in concentration is not in equal amount for every substance as their molar coefficients are different. Therefore, when species A change by 2 moles, species C will change by 4 moles.
Although the change in A’s concentration (2 moles <-> 2 moles) and change in C’s concentration (4 moles <-> 4 moles) are same, the change in A’s concentration does not equal to the change in C’s concentration (2 does not equal 4).
The reason for this is because molar ratio between the species are FIXED for each particular chemical reaction. So, although all species do not necessarily change by the same amount (in terms of moles), each species’ change in concentration is constant and consistent with their mole coefficient.
Equal and constant change in concentration mean completely different things!
Do not get them mixed up.
Also, note that the examples above are examples of dynamic equilibrium NOT static equilibrium reaction.
We will compare the similarities and differences between static and dynamic equilibrium in the next learning objective.
If you do not completely understand this, you can watch the lecture video at the end of this week’s notes. We will have a visual diagram illustrating this to help with clarification.
Learning Objective #2 – Distinguish between static and dynamic equilibrium.
Since we have already touched on the term ‘equilibrium’, we might as well continue on with it.
Did you know that you may lose a mark if you do not provide a definition for the main keyword in exam questions?
Anyways, continuing on with our learning objective, let’s examine some definitions.
Dynamic equilibrium is an equilibrium where reactants are converted into products (and vice versa) at a constant rate (constant rate of change).
This was already explored in the prior learning objective in the example involving compounds A, B, C and D.
Like dynamic equilibrium, static equilibrium is another type of equilibrium. So, when writing out the chemical reaction expressions for dynamic and static equilibrium, you would expect to write the equilibrium symbol.
With static equilibrium, there is little to no chemical change between reactants and products converting back and forth like you saw with dynamic equilibrium.
None of those compounds are being converted back and forth at a molecular level, so NO chemical bonds are being broken or formed at a molecular level for systems at static equilibrium.
Thus, you obviously cannot observe any visual changes in static equilibrium as there is no actual change even at the molecular level.
So a definition of static equilibrium is an equilibrium where both reactants and products are no longer converting between each other.
So, the rate of change of the forward AND reverse reaction are effectively zero.
Note that: Although the rate of the forward and reverse reaction are both zero, the forward rate of reaction is STILL EQUAL to the reverse rate of reaction (similar to dynamic equilibrium).
Why? Well, 0 = 0 means they’re equal.
In dynamic equilibrium, the rate of the forward reaction is not zero and the rate of the reverse reaction are NOT zero. This is because in the forward reaction, the reactants are being interacting and being converted into products. Therefore, the forward rate of reaction cannot be zero. Vice versa, in the reverse reaction, the product molecules also react with each other and are being converted into reactants. Therefore, the reverse rate of reaction cannot be zero.
Note that: As mentioned previously, the rate of the forward and the rate of reverse reaction in dynamic is also equal.
You may ask, why are static equilibrium called chemical reactions then?
Analogy: Well, think of a sea-saw in a park. The sea-saw may have been initially moving as children were playing on it. This means that the sea-saw was at dynamic equilibrium. Then the children got off the sea-saw. Eventually, after a while, the sea-saw stops moving. The sea-saw is now at static equilibrium.
Net force applied onto or experienced by the system (sea-saw) is zero.
This means that the rate of forward reaction is zero and rate of reverse reaction are both equal to zero. Static equilibrium is typically seen in real life rather than in chemistry.
This is because most reactions are either complete (‘non-reversible’) or at equilibrium (‘reversible’). For those that reactions that are at equilibrium, most of them are at dynamic equilibrium.
We will explore the terms non-reversible and reversible used to describe chemical reactions shortly.
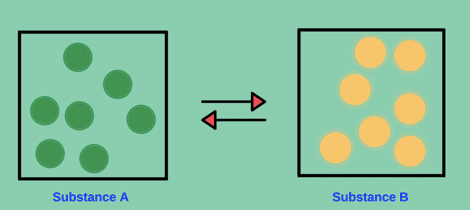
A generic example of a STATIC EQUILIBRIUM can be expressed as the following:
Substance A ⇌ Substance B
Since no substance A are being converted into substance B atoms, the rate of the forward reaction is zero and rate of reverse reaction is also zero.
Molar ratio has NO effect on the change in concentration levels (amount) for species in static equilibrium. This is because the species are not being converted between each other (A are not being converted into B and B are not being converted into A).
Let me illustrate static equilibrium at a molecular level below:
You can sit and watch the atoms of substance A and B. But, 100 years from now, none of substance A’s atom will be converted into an atom B or vice versa.
The equilibrium can be considered very close to or effectively static.
As you have learn in the Year 11 HSC Chemistry Syllabus, graphite and diamond are allotropes. Both contain only carbon atoms.
But did you know that diamond can be transformed into graphite (more stable carbon form) if you give diamond a billion years with the kinetic energy at room temperature?
Vice versa, you can also transform graphite into diamond provided you supply enough heat energy and pressure.
The Diamond ⇌ Graphite is a static equilibrium at room temperature (25 degrees celsius).
It takes so long to convert diamond into graphite, you assume that there is no chemical change occurring at the molecular level at room temperature.
Let’s recap by comparing the SIMILARITIES and DIFFERENCES between STATIC and DYNAMIC equilibria:
Similarities:
The rate of the forward and reverse reaction are equal.
This would mean that the change in each species’ concentration is constant, e.g. if 2 moles of A is being consumed and reacted to form products, x moles of product will react to form 2 moles of A again. The change in A’s concentration is constant and consistent with reaction’s mole ratio if reaction is at equilibrium.
Difference:
Static equilibria: The rate of the forward reaction is zero and the rate of the reverse reaction is also zero. (Equal as 0 = 0).
Generally, static equilibria can occur in closed systems.*
In extreme or rare conditions, such as deep below the earth’s crust, static equilibrium can exist in open systems. But, for HSC Chemistry purposes, it’s can be assumed that equilibrium can only be established in closed systems.
Static equilibrium can be considered irreversible.**
Dynamic equilibria: The rate of the forward reaction is NOT zero and the rate of the reverse reaction is also NOT zero (Equal e.g. 2 = 2 but not 0 = 0).
Generally, dynamic equilibria can occur in closed systems.*
Dynamic equilibria are reversible.**
Notes to asterisks:
*We will examine what open and closed systems are shortly.
** We will explore the definitions of irreversible and reversible reactions shortly.
What are reversible and irreversible reactions?
Reversible reactions are reactions where reactants molecules can react with each other to form products and vice versa.
If this reversible reaction occurs in a closed system, a dynamic equilibrium will be established or reached after some time.
We will explore what a closed system actually is very shortly, so bare with us for the time being.
Example of reversible reaction: N2(g) + 3H2(g) ⇌ 2NH3(g) + Heat
Note that: a chemical reaction such as the one above has potential to establish dynamic equilibrium. However, if it occurs in an open system rather than a closed system, equilibrium may never be reached or established.
We will explore why this is the case when we look into open and closed systems very shortly.
Irreversible reactions are reactions where reactants react together to form products, however, the products cannot react with each other to create the reactants.
The reaction pathway is one directional.
Example of irreversible reaction: 2Mg(s) + O2(g) + Heat -> 2MgO(s)
Please note the difference in chemical signs between reversible and irreversible reactions used in the above examples. Reversible reactions are denoted with double, reverse arrows whereas irreversible reactions have a single-way arrow.
Now, you may ask, so are static equilibrium reactions never reversible?
Technically, no. Like all irreversible reactions, they are technically reversible. If significant amounts of energy is required to overcome the bond energy or energies break the chemical bond(s) of the product in order to convert the products back into reactants, we deem such chemical reaction as irreversible.
Why? Well, it is very energy and cost inefficient to reverse the chemical reaction.
Under normal conditions, the activation energy barrier that products need to overcome to be converted into reactants is very high in irreversible reactions. As a result, not enough of the products are being converted back into reactants at an amount that is measurable.
Therefore, it will be more energy and cost efficient to obtain the reactant by manufacturing it rather than breaking the chemical bonds of the product to obtain the reactant.
For example, the Diamond <-> Graphite static equilibrium requires such much energy to convert from one to another that it is considered irreversible.
Dynamic equilibrium reactions are reversible by definition.
Static equilibrium can be considered irreversible by the definition of products cannot be converted into reactants (irreversible) at equilibrium as the rate of reaction is zero.
Closed systems vs Open systems
So, we mentioned the term ‘closed system’.
If there is a closed system, there must be an open system. What do the two terms mean and their differences?
A closed system is an environment that DOES NOT involve the exchange of matter with its external environment.
Therefore, in a closed system, all chemical reactions only involves the species belonging in the system.
While a closed system cannot exchange matter with surrounding environment, it CAN exchange energy with the surrounding (external environment).
An open system is an environment that exchanges matter AND energy with its ambient, external environment.
Dynamic equilibrium only occurs in closed systems because only closed systems will prevent matter from the environment from interacting with the matter in the system due to the presence of some barrier. Therefore, this allows the chemical reaction occurring in a closed system to establish dynamic equilibrium where the forward reaction is equal to the reverse reaction.
If a dynamic equilibrium occurs in an open system, equilibrium is likely to never be achieved as the concentrations of the reactants and products are consistently being varied. We will explore more into this when we examine Le Chatelier’s Principle in Module 5 – Inquiry Question #2 notes.
Comparatively, static equilibrium could exist in both open and closed systems.
For example, the Graphite <-> Diamond static equilibrium can exist in both open and closed systems.
However, for HSC Chemistry purposes, we can assume that equilibria can only be established in a closed system.
===
Fun fact: There is another form of system called isolated system.
In an isolated system, the matter within that system CANNOT exchange matter nor energy with the surrounding. A reaction occurring within a perfect insulator can be classified as an isolated system.
Learning Objective #3 – Analyse the reversibility of chemical reactions!
Are the following reactions reversible?
Reaction #1: Cobalt (II) Chloride Hydrate and cobalt (II) chloride dehydrate
Chemical equilibrium reaction: Co(Cl)2•6H2O(s) -> [Co(H2O)6]2+ (aq) + 4Cl–(aq) ⇌ [CoCl4]2- (aq) + 6H2O(l)
Note: 4Cl– is used instead of 2Cl–. This is because we need to add chloride ions to form the [CoCl4]2- ion as seen in step 2 in procedure below (adding chloride ions to see if reaction proceeds to the right).
So, how do you determine whether the above equation is reversible?
Well, we can use colour change to determine whether or not the chemical reaction is reversible. If it is reversible, it is a dynamic equilibrium reaction!
We can justify why we use colour change to determine whether it is an equilibrium because there is a unique colour for substances on the reactant and product side in Reaction #1. These colours are the following:
Co(Cl)2•6H2O = Solid crimson-coloured crystals.
[Co(H2O)6]2+ = Pink-colour ion in solution.
[CoCl4]2- = Blue-colour ion in solution.
So, if we can convert the solution from pink to blue and from blue to pink (or vice versa), it means that we reversed the chemical reaction!
To determine whether the above reaction is reversible, we can first add heat to decompose the crimson crystals into a pink solution which can turn blue if there is high concentration of chloride ions in solution.
If the reaction is reversible, we should be able to convert the blue solution back into the pink solution. The reason to why such colour change can happen will be explored in Module 5 – Inquiry Question #2 notes where we talk about Le Chatelier’s Principle and collision theory.
How will you do this experiment in your school laboratory?
Step 1: Weigh and dissolve 0.35 grams of solid cobalt (II) hexahydrate in a beaker of 20ml of water.
Step 2: Add chloride ions (e.g. in the form of HCl(aq)) into the solution. Observe for colour change. (You should see pink solution turning blue). This is because the [Co(H2O)6]2+ ion is being converted into the [CoCl4]2- ion with the addition of chloride ions.
Step 3: Add 10ml of water and observe for colour change. (You should see the blue solution turning into pink) solution. This is because the [CoCl4]2- ion is being converted into the [Co(H2O)6]2+ ion.
Ta da! We have just observed a reversible reaction by observing colour changes.
Note that if the colour of the [CoCl4]2- ion and the [Co(H2O)6]2+ ion are the same, we cannot use colour changes to determine if the reaction is reversible or not as we won’t see any colour changes.
Conclusion: Reaction #1 is reversible!
Again, the reason why adding water to the blue solution will turned the solution into pink (colour change) can be explained using collision theory or predicted using Le Chatelier’s Principle. We will go explore that in the next set of notes.
Recap: The equilibrium reaction for reaction #1 is: [Co(H2O)6]2+ (aq) + 4Cl- (aq) ⇌ [CoCl4]2- (aq) + 6H2O(l)
Do note that if the above equilibrium reaction is written exactly as above, the forward reaction is endothermic. That means, if you add heat, the solution will turn from pink to blue. So, instead of adding chlorine ions, this means that we can also use temperature to determine if the reaction #1 was reversible or irreversible.
There are also many other methods to determine if reaction #1 is reversible or not. For example, adding silver nitrate to precipitate the chloride ion will turn solution from blue to pink. Again, we will explore the reason for why changing temperature and adding reagents can allow us to see such colour change in the next week’s notes following this.
Alternative Experiment: At school, you may also determine the reversibility of cobalt (II) chloride hexahydrate – cobalt (II) chloride dehydrate reaction use Cobalt (II) chloride paper strips.
These cobalt (II) chloride paper strip have the formula, CoCl2(s),which is blue. When they are exposed to moisture in the air, they turn pink. There is water vapour in the air which the cobalt (II) chloride paper strip can bond with. This gives us the formula: Co(Cl)2•6H2O which is what we used as the starting product and thus the paper will look pink.
Overall reaction: CoCl2(s) + H2O (g) <-> Co(Cl)2•6H2O(s) ; in this experiment, water is in gaseous state as it exist as water vapour in air.
Drying the cobalt (II) chloride paper strip will turn the pink colour into blue colour again (e.g. using a hair blowdryer).
Alternatively, at school, you could dip the cobalt (II) chloride strips into a beaker of water. In such case, the blue strip will turn pink. You will then dry the cobalt (II) chloride strips (e.g. using a hair blowdryer) and it will turn blue again.
Overall reaction: CoCl2(s) + H2O (l) <-> Co(Cl)2•6H2O(s) ; in this experiment, water exists as liquid state in the beaker.
Now, let’s explore:
Reaction #2: Iron (III) nitrate reacting with potassium thiocyanate
Chemical Equilibrium Reaction: Fe3+ + SCN– ⇌ FeSCN2+
Please note that potassium and nitrate ions reacted to form KNO3 in solution. They are simply spectator ions that would not contribute towards determining the direction in which the chemical equilibrium will shift for Reaction #2.
Both potassium and nitrate ions are also both colourless in solution.
We included colourless water in the Reaction #1’s equation because in Reaction #1 we added water as part of experimental procedure.
Adding water helped shift the chemical equilibrium position towards the left in favour of the reverse reaction, turning the blue solution into pink.
Anyways, let’s come back to Reaction #2.
Here in reaction #2, we will not increase or decrease the concentration of potassium or nitrate ions as the ions will not affect the direction (left/right) in which the equilibrium system will shift in favour towards.
Hence, we exclude them out of the chemical equilibrium equation.
Again, since there are distinctive colours for each of the ions in Reaction #2, we can use colour changes to assess the reversibility of the reaction.
If a colour change can be imposed and reversed, it will be a reversible, dynamic equilibrium reaction!
Fe3+ = Yellow colour in solution.
SCN– = Colourless colour in solution.
FeSCN2+ = Crimson colour in solution.
When equilibrium has been established, the solution will be crimson due to the presence of FeSCN2+ ions
Depending on the change in the red colour’s intensity, we can determine whether or not the reaction is reversible and, if so, which direction the equilibrium has shifted towards.
If the crimson solution reduces in red intensity (yellow colour increasing in intensity due to formation of Fe3+ ions in solution), it means that the equilibrium has shifted to the left. Therefore there would be drop in the concentration of FeSCN2+ ions but increase in the Fe3+ and SCN– ions in solution.
Vice versa, if the crimson colour intensifies it would mean that the reaction has shifted to the right, increasing the concentration of FeSCN2+ ions and decreasing the concentration of thiocyanate (SCN-) and iron ions.
The forward reaction of Reaction #2 to form iron (III) thiocyanate ion is endothermic. Therefore, the reverse reaction is exothermic.
If you do this experiment at school, adding Fe3+ and SCN- ions in solution to form iron (III) thiocyanate ion. Then you can change the temperature of the system to shift the equilibrium position in favour of the forward or reverse reaction.
For example, if you increase the temperature, the equilibrium position will shift to the left so the concentration of iron (III) thiocyanate will decrease and Fe3+ ion will increase. This means that the solution will decrease in red colour intensity or be yellow.
Vice versa, if you decrease the temperature, the equilibrium position will shift to the right so the concentration of iron (III) thiocyanate will increase and Fe3+ ion will decrease. This means that the solution will increase in red colour intensity or not longer be yellow.
The point here is that since the colour of the system can be reversed, reaction #2 is reversible.
In next week’s notes, we will explore Le Chatelier’s Principle to predict and explain the direction in which an equilibrium position will shift.
So, don’t worry right now if you don’t understand why the increasing temperature in Reaction #2 shifted the equilibrium position to the left.
Conclusion: Reaction #2 is reversible!
Alright! Let’s move on to exploring another reaction, the burning of magnesium metal!
Reaction #3: Burning Magnesium!
Chemical Reaction: 2Mg(s) + O2(g) -> 2MgO(s)
Magnesium metal is being heated in the presence of oxygen gas (i.e. in the presence of air), forming magnesium oxide solid (MgO).
The energy required to break the Mg-O bond and convert the MgO back into pure magnesium would be ENORMOUS.
It is so not worth reversing such reaction that you will be much better off go mine for pure magnesium instead.
Another way to think about this: Say you burn a piece of paper down to its ashes, would you spend efforts putting it back together?
Just imagine the time and effort you need to reverse Reaction 3. It is not doable.
All burning reactions are considered irreversible.
Another way to think about it! Under normal conditions (e.g. not in very high temperatures), the activation energy barrier that products need to overcome to be converted into reactants is very high in irreversible reactions. As a result, not enough of the products are being converted back into reactants at an amount that is measurable.
Conclusion: Reaction #3 is NOT reversible.
Lastly,
Reaction #4: Burning Steel Wool
We talked about how all burning reactions are considered irreversible.
So, we know that our conclusion will be that Reaction #4 is irreversible!
However, we will explore the burning of steel wool in terms of its chemical equation as it is examinable.
Chemical Reaction: 4Fe(s) + 3O2(g) -> 2Fe2O3(s)
Heating the steel wool (mainly iron) in the presence of oxygen gas, forming Ferric Oxide. Burning is a permanent chemical change and cannot be reversed. Atoms are rearranged, new bonds formed e.g. the Fe-O bonds in Ferric Oxide.
In order to reverse the reaction, we need to break the Fe-O bonds to free the Fe atoms. The Fe-O almost qualified as an ionic bond by definition, meaning that the Fe-O bond has very high bond energy.
Because of the high amount of energy required to break the Fe-O bond and difficulty to reverse such reaction under normal circumstances, the burning of steel wool is irreversible.
Learning Objective #4 – Analyse examples of non-equilibrium systems in terms of the effect of entropy and enthalpy.
Before we examine some NON-equilibrium systems (complete reactions), let’s explore what is meant by entropy and enthalpy. We have already learnt this in HSC Preliminary Course so this will serve as a recap.
Entropy
Entropy, denoted by the letter ‘S‘, refers to the number of ways or states that energy can be distributed to the species (particles) in the system.
The greater the number of state that the species can exist in a system, the more entropy the system has.
A system’s entropy can be increased in the following ways:
Increasing the number of species in the system
Increasing the system’s total energy (e.g. by increasing temperature, thus, increasing kinetic energy)
Increasing the system’s volume
It is important to note that all particles have some form of energy such as chemical bond energy and kinetic energy. By increasing the number of particles in a system, the number of ways in which energy is distributed throughout the system increases. Thus, entropy of the system increases.
Similarly, by increasing the system’s total energy, (e.g. by increasing temperature or kinetic energy), average kinetic energy possessed by particles in the system increases. Thus, more collisions will occur and so more particles are able to interact and exchange their energy. This will therefore increase the number of ways in which energy is distributed throughout the system. Thus, entropy of the system increases.
By increasing the system’s volume, there will be an increased number of ways, due to increased space, in which particles can be distributed throughout the system. Since all particles have some form of energy such as chemical bond energy and kinetic energy, this increases the number of ways in which energy is distributed throughout the system. Thus, the entropy of the system increases.
Enthalpy
Enthalpy, denoted by the letter H, refers to the total internal energy present inside a system.
You may have seen ‘ΔH‘. It means change in enthalpy. The ‘Δ’ symbol means change.
Hence, ΔH refers to the total change in internal heat energy that is gained or loss by the system.
The following thermodynamics equations will be useful later on
(In addition to the formulas learn in the Preliminary HSC Chemistry Course!)
Entropy Formulas:
Change in a system’s entropy = Entropy of products – Entropy of reactants
ΔS (system) = ΔS (products) – ΔS (reactants)
Change in the surrounding’s entropy = Change in enthalpy of reaction (Joules) / Temperature of reaction (Kelvins)
ΔS (surrounding) = ΔH (reaction) / T
Total change in entropy = Change in system’s entropy + Change in surrounding’s entropy > 0
ΔS (total) = ΔS (system) + ΔS (surroundings) > 0
Enthalpy Formulas:
Change in internal energy = heat energy in system + work done by the system.
ΔU = q + w
At constant pressure, there is no work done by the system on the particles (w=0)
Hence change in internal energy (ΔU) = heat energy of system (q)
At constant pressure:
Change in enthalpy = Change in internal energy + pΔV
ΔH = ΔU + pΔV
But since no work is done at constant pressure, ΔU = q
So, ΔH = q + pΔV
How do you find pΔV?
pΔV = ΔnRT
Where:
n is the moles of ideal gas
R is the ideal gas constant (8.314 J/K/mol)
T is the temperature in kelvins
ΔH = H(product) – H(reactant)
Analysing the chemical equation in terms of entropy and enthalpy
Recap from preliminary course:
When determining the change in entropy, the states of matter have greater priority than the molar ratios between species (reactants and products) in the reaction.
In order of decreasing entropy: Gases, Aqueous solutions, Liquids, Solids.
Hence, gases have the greatest entropy out of all states of matter.
If there are gases on both sides of the chemical equation, we need to use molar ratios.
Analysing photosynthesis in terms of entropy and enthalpy
Chemical equation for photosynthesis: 6CO2(g) + 2H2O (l) -> C6H12O6(s) + 6O2(g)
NOTE: Glucose appears as solid because that’s how glucose exists as standard state (assuming standard state is referring to 25 degrees celsius and not referring to 0 degree celsius. As usual, the pressure of reaction should occur at 1 atmospheric pressure or 100 kPa.
What can we say about the change in the system’s entropy for photosynthesis reaction?
Answer: It is negative. Both side has gas. Both side has 6 moles of gas (6 moles of carbon dioxide and 6 moles of oxygen). However, the reactant side has a liquid and the product side has a solid. The molar ratios of the liquid and solids do not matter as liquid has greater entropy than solids. Therefore, the system’s (plant) entropy decreases after performing photosynthesis.
What can we say about the change in the surrounding’s entropy?
Answer: Positive. You are NOT expected to know this but, be aware, that the TOTAL change in entropy has both SYSTEM and SURROUNDING entropy components.
What can we say about the change in the system’s enthalpy?
Answer: Positive. If the change in enthalpy is negative, it means that the reaction is exothermic. This means, overall, heat has been lost from the system and the surrounding environment gains temperature. If the change in enthalpy is positive, it means that the reaction is endothermic. Photosynthesis absorbs energy from the sun, it is an endothermic reaction. Hence, the change in enthalpy is positive.
Analysing combustion reactions in terms of entropy and enthalpy
Example #1: C3H8(g) + 5O2(g) -> 3CO2 (g) + 4H2O(g)
NOTE: For combustion reactions, the water product will appear as a gas (water vapour) if the exam question states that the combustion occurs at temperatures greater than 100 degrees celsius. If standard temperature is given (25 degrees celsius) or NO temperature is given, you should write water as a liquid.
For example #1, what can we say about the change in the system’s entropy?
Answer: 6 moles of gas on the reactants side vs 7 moles of gas on the products side. Change in the system’s entropy is positive
For Example #1: what can we say about the change in the system’s enthalpy?
Answer: Heat is lost from the system and distributed into the surroundings for combustion reactions. All combustion reactions are exothermic. Change in enthalpy of system is therefore negative.
Example #2: CH3OH(l) + 3/2 O2(g) -> CO2(g) + 2H2O(g)
For example #2, what can we say about the change in the system’s entropy?
Answer: 1.5 moles of gas on the reactants side vs 3 moles of gas on the products side. The change in the system’s entropy is positive.
For example #2, what can we say about the change in the system’s enthalpy?
Answer: Same explanation as example #1, all combustion reactions are exothermic. Change in system’s enthalpy is negative.
Example #3: C8H18(l) + 17/2 O2(g) -> 9H2O(g) + 4CO(g) + 2CO2(g) + 2C(s)
For example #3, what can we say about the change in system’s entropy?
Answer: 8.5 moles of gas on the reactants side vs 15 moles of gas on the products side. Change in system’s entropy is positive.
For example #3, what can we say about the change in the system’s enthalpy?
Answer: Same explanation as for example #1, all combustion reactions are exothermic. The change in enthalpy is negative.
Learning Objective #5 – Investigate the relationship between collision theory and reaction rate in order to analyse chemical equilibrium reactions
Reaction rate (rate of reaction) can be thought as of how fast reactants are being turned over and converted into products in a set period of time.
There are many factors that affect reaction rate. Some common ones are:
Concentration of reactants
Temperature of the system
Pressure of the system
Surface area of reactants
Activation energy of the chemical reaction (whether or not a catalyst is used in the reaction)
The collision theory adds to our understanding of reaction rates in terms of the number of successful collisions between species involved in a chemical reaction.
If there is no successful collision, there will be no reaction occurring and thus the reaction rate will be zero. This is why collision theory is important.
This is also the relationship between collision theory and reaction rate (number of successful collisions). That is, collision theory tells us that the larger the number (frequency) of successful collisions, the faster the rate of reaction.
But what specifically does the collision theory tell us in terms of what a ‘successful collision’ mean?
Well, the two intrinsic properties that are possessed by atoms, molecules, compounds and ions (mentioned in the collision theory) can help explain. The two properties are the following:
1. The collision energy of the reacting species must be greater than or equal to the required activation energy for the chemical reaction to occur.
Without sufficient collision energy, existing bonds will not break to form new bonds for the re-arrangement of atoms and, subsequently, convert reactants into products. In equilibrium, product molecules will also need to have collision energy that is equal or greater than activation energy to be converted into reactants.
2. The collision orientation of the reactant species will determine whether or not a chemical reaction will take place as well. The reactants needs to collide at an effective orientation to be converted into products. In equilibrium, products will also need to collide at an effective orientation to be converted into reactants.
The two intrinsic properties MUST BOTH BE MET in order for a successful collision to occur and thus for a chemical reaction to place as per collision theory. If only one of the two (or none) criteria is met, the rate of reaction will be zero.
ILLUSTRATION OF EFFECTIVE AND INEFFECTIVE COLLISION ORIENTATION
Example of chemical reaction: Oxygen gas reacting with carbon monoxide to form carbon dioxide.
Below is a diagram of INEFFECTIVE collision orientation between oxygen gas and carbon monoxide resulting in NO products being formed.
Now let’s take a look what an EFFECTIVE collision orientation may look like:
Effective collision orientation results in the following:
The O-O bond of the oxygen molecule being broken.
With effective orientation collision orientation, the C-O bond can be formed between oxygen gas and carbon monoxide, resulting in carbon dioxide product.
Note that the left over oxygen from the initial oxygen diatomic molecule will form a bond with another oxygen. This is because oxygen is more stable in its diatomic state than existing as a single atom in nature.
Hence, a new oxygen diatomic molecule is formed as a by-product.
If you were to measure the reaction rate for this chemical reaction, you can measure the rate of carbon dioxide formation or drop in carbon monoxide concentration.
Analysing chemical equilibrium reaction using collision theory and reaction rate
As outlined in the above paragraph, in a closed system, making adjustments to the following will have an effect on the rate of reaction in a chemical equilibrium:
Concentration of reactants or products
Changing the temperature of the system
Pressure of the system
Volume of the system
Activation energy of a chemical reaction (using or not using a catalyst)
NOTE: We will NOT explore how each of the above factor will cause an equilibrium reaction will shift in favour towards (forward/reverse reaction) in this learning objective. Instead, we will explore it in next week’s notes, Module 5 – Inquiry Question #2.
So, in this learning objective, we will only make you aware of how each factor can affect the collision energy and/or the effective collision orientation of reacting species. Then, in next week, we will explore how each factor affects which way the system’s equilibrium position will shift towards.
Changing the temperature of the system
Let’s start off by examining the effects of the change in temperature of a closed system.
Increasing temperature will increase the kinetic energy of the reactants and products participating in the chemical equilibrium reaction.
As reacting species collide, kinetic energy is converted into collision energy. Thus, an increase in kinetic energy in reacting species will increase their collision energy. This means that more of the reacting species will have collision energy that is greater or equal to the activation energy required for the chemical reaction to proceed (successful chemical reaction to occur).
Therefore, this would result in an increased number of successful collisions and increased rate of reaction.
This is true for both one directional and reversible chemical reactions. We will explore how changing the temperature of the system will affect equilibrium reactions in next week’s notes.
Note that in equilibrium reactions, if the forward reaction of a chemical reaction is endothermic, this would mean that the reverse reaction would be exothermic (or vice versa). This is due to the law of conservation of energy.
In next week’s notes, we will explore how changing the temperature of the system will favour either forward or reverse reaction using collision theory and Le Chatelier’s Principle.
Changing the pressure of the system
(Pressure is a factor that only affects gases; NO effect on solids, liquids and aqueous substances)
Now, it is time to explore effect of increasing pressure.
Please note that increasing or decrease in pressure only affect gas reactants and products in the chemical equilibrium reaction.
Increasing pressure increases the proximity of gas molecules and, thus, increases the frequency they collide & interact with each other.
This increased interaction raises the possibility of effective collision orientation for a given period of time.
The result is increased rate of reaction – again, temporarily shifting the equilibrium position due to increased pressure.
Changing the volume of the system
(Volume is a factor that only affects gases; NO effect on solids, liquids, aqueous)
It is important to realise that pressure and volume are inversely related.
Hence, increasing the pressure of a system has the same effect as decreasing the volume of the system, in terms of shifting the equilibrium reaction towards one (same) side of the chemical equation.
For example, lowering the volume of a system would increase the proximity of gas molecules and, thus, increases the frequency they spend colliding & interacting with each other.
This increased interaction between substances (reactants and/or products) in the equilibrium raises the possibility of effective collision orientation for a given period of time.
Overall, the rate of reaction would increase and temporarily shift the equilibrium towards one side of the chemical reaction to minimise the disturbance.
Please watch the lecture recording at the end of this week’s note if you do not understand how pressure and volume are inversely related. I use a visual diagram in the video recording to aid your understanding.
Changing the concentration of reactants and products participating in the chemical equilibrium reaction
Finally, we explore the effect of increasing the concentration of the reactants and products of a equilibrium reaction.
Well, by now, you should have figured out that increasing the number of reactants and/or products would increase the number of atoms. This would increase the frequency of collisions between species as well as increasing the probability for reacting species to collide at an effective orientation. This means that more reacting species will satisfy the collision theory and so more successful collision will occur. This will increase the rate of the overall reaction.
NOTE:
The decrease in pressure, volume, concentration of species can be asked in exams. However, the effects are just shifting the direction (left or right) of equilibrium reaction to the other direction. We will cover which direction an equilibrium will shift in the next set of notes using collision theory and Le Chatelier’s Principle.
Adding a catalyst
A catalyst provides an alternative pathway in which reactants in the chemical reaction can undertake to form products and vice versa! This alternative reaction pathway has a lower activation energy than when the chemical reaction is to proceed without a catalyst.
This means that less energy is required to break chemical bonds necessary for the reactants to form products and vice versa (breaking the bonds in the products to form reactants in equilibrium). This means that the total number of successful collisions on the reactant and products side of the equilibrium reaction have both been increased by the same amount.
Therefore, the time required for the chemical reaction to reach equilibrium will be lowered. However, catalysts do not affect the position of equilibrium as both the rate of forward and reverse reaction are increased by the same amount.
Side Note: Since catalysts do not change the value of free Gibbs energy, catalysts does not catalyse non-spontaneous reactions and only spontaneous reactions.
===
Fun Fact: You will encounter Arrhenius’s equation in Module 5 – Inquiry Question and which will provide the mathematical explanation of how lowering the activation energy of the reaction will increase the rate of the forward and reverse reaction. Note that this equation only applies to reacting species that are simple and not complex in nature.
Week 1 Homework Set (Essential for Band 5)
Question 1: Hydrogen reacts with iodine to form hydrogen iodide. But how are hydrogen and iodine gases reformed in this reaction? Explain. [3 marks]
Question 2: Explain how the collision theory and rate of reaction are related. [4 marks]
Question 3: Describe what it means when the change in concentration is constant. [3 marks]
Question 4: Describe the difference between static and dynamic equilibrium reactions. Provide an example for each. [6 marks]
Question 5: Explain why entropy is an important measure for chemical reactions. [3 marks]
Question 6: Explain how activation energy differs from from collision energy. [4 marks]
Question 7: Explain the inverse relationship between volume and pressure and how it affects reaction rate and entropy. [6 marks]
Question 8: Describe the features of an open and closed system and provide an example for each. [6 marks]
Question 9: Explain what is meant by enthalpy for chemical reactions and the significance of the sign of enthalpy? [4 marks]
Curveball Questions (Moving from Band 5 to Band 6!)
Curveball Question #1: Explain what is meant by a ‘process is at steady state’ ? Provide an example. [3 marks]
Curveball Question #2: Explain the difference between constant change in concentration and equal change in concentration. Use chemical equations to support your answer. [6 marks]
Curveball Question #3: How does increasing and reducing surface area of reactants affect the rate of reaction? [4 marks]
Curveball Question #4: Is it possible to have positive total entropy with a negative system entropy? If so, provide an example of such scenario. [3 marks]
Curveball Question #5: Is it possible to have negative surrounding entropy but positive system entropy? If so, provide an example of such scenario. [3 marks]
Curveball Question #6: Define temperature and kinetic energy. How are these two terms related by definition? [3 marks]
Solutions to Week 1 Homework Set
Solution to Question 1:
The reaction between hydrogen and iodine can be written as follows:
H2 (g) + I2 (g) <-> 2HI (g)
Hydrogen iodine (HI) molecules react with each other to reform hydrogen and iodine gas.
Marking Criteria:
1 mark = Chemical equation
1 mark = Explaining how H2 and I2 gases are formed.
Solution to Question 2:
Collision theory is a theory that explains how the reacting species must have both effective orientation and sufficient collision energy (2 criteria) in order for a chemical reaction to take place.
The rate of reaction refers to the amount of reactants being converted into products for a set period of time.
If the two criteria of the collision theory are not satisfied, there will be a zero rate of reaction. This is because no reactants are being converted into products. Moreover, the amount of reacting species that satisfy (or does not satisfy) the two criteria will have an effect on the overall rate of reaction.
Marking Criteria:
1 mark = Definition of Collision Theory
1 mark = Definition of Rate of Reaction
2 marks = explaining (cause & effect) the relationship between collision theory and rate of reaction
Solution to Question 3:
Concentration of a substance is the number of moles (particles) of the substance per one litre of volume. A constant change in concentration means that the concentration of the species involved in the reaction (products and reactants) are increasing or decreasing according to their chemical coefficients in the chemical equation.
Marking criteria:
1 mark = definition of concentration
2 marks = definition of constant change in concentration relating to species’s individual chemical coefficient
Solution to Question 4:
A static equilibrium reaction refers to an equilibrium where both reactants and products are no longer converting between each other. So, the rate of the forward and reverse reaction is effectively zero.
An example of a static equilibrium is diamond turning into graphite. So much energy is required to convert diamond into graphite in a short period of time and vice versa that it is considered a static equilibrium and irreversible.
A dynamic equilibrium reaction refers to an equilibrium reaction where the reactants are converted into products and the products species are converted into reactants at a constant rate.
An example of a dynamic equilibrium is the Haber Process: N2 + 3H2 <-> 2NH3
Marking criteria:
1 mark = definition of static equilibrium
1 mark = definition of dynamic equilibrium
1 mark = example of static equilibrium
1 mark = example of dynamic equilibrium
Solution to Question 5:
Entropy refers to the number of ways or states that energy can be distributed to the species (particles) in the system.
Entropy allows us to predict whether a reaction will occur spontaneously. For example, an exothermic reaction with increasing entropy would generally occur spontaneously. This is because of the change in Gibbs free energy is negative. (Year 11 course)
Figuring out the change in Gibbs free energy is only valuable under fixed temperature and pressure. However, as these conditions can be established in real life closed systems, it allow chemist to establish and monitor spontaneous chemical reactions to produce things for everyday use! For example, the Haber Process mentioned earlier, N2 + 3H2 <-> 2NH3, used to produce ammonia (bleach) occurs at fixed temperature and pressure.
Marking criteria:
1 mark = definition of entropy
1 mark = relationship between entropy and spontaneity of chemical reactions
1 mark = explaining the importance of spontaneity of chemical reactions
Solution to Question 6:
Activation energy refers to the minimum energy required by reactants to be converted into products (and vice versa for dynamic equilibrium reactions), that is, the energy required to reach the transition state by breaking the necessary chemical bonds. Collision energy refers to the kinetic energy that is converted when reacting species collide with each other.
Sufficient collision energy must be present to break the chemical bonds required for the reactants to be converted into products (or vice versa) for a chemical reaction to take place. If the reaction only requires one chemical bond to be broken, then activation energy is equal to the bond energy of the chemical bond that is required to be broken. Usually, more than one chemical bond is required to be broken though.
Every species in a reaction would have some amount of collision energy. However, this collision energy may not be greater than or equal to the required activation energy for the species to react and form product(s).
Marking criteria:
1 mark = Definition of activation energy
1 mark = Definition of collision energy
2 marks = Relationship between activation energy and collision energy
Solution to Question 7:
Entropy refers to the number of ways or states that energy can be distributed to the species (particles) in the system.
The rate of reaction refers to the amount of reactants being converted into products for a set period of time.
When you decrease the volume of the system, you will increasing the pressure of the system. This is because particles in the system will be spending more time colliding with each other and the walls of the system. This means, for a given period of time, there will be an increased number of reacting species colliding with each other, this increases the rate of reaction.
In terms of entropy, decreasing the volume will reduce the number of ways in which particles (and thus energy) can be distributed in the system. Hence, decreasing a system’s volume will decrease the entropy of system. It is important to note that all particles have some form of energy, such as chemical bond energy and kinetic energy.
Vice versa, if you increase the volume of the system, you will be decreasing the pressure of the system. This is because the particles will spend less time colliding with each other and with the walls of the system. Thus, since there is less collisions between reacting species for a given period of time, the rate of reaction decreases.
In terms of entropy, increasing the volume will increase the number of ways in which particles (and thus energy) can be distributed throughout a system. Hence, increasing a system’s volume will increase the entropy of system.
Marking criteria:
1 mark = define rate of reaction
1 mark = define entropy
2 marks = inverse relationship between increasing/decreasing volume and pressure.
2 marks = explain how increasing/decreasing volume and pressure affect reaction rate and entropy
Solution to Question 8:
An open system is one where the external environmental factors such as temperature, pressure, volume may affect the concentration of species that are participating in a chemical reaction in the system. This is because the substances in the system interact with matter in the surrounding.
An example of an open system is a cup of coffee.
Comparatively, a closed system is a closed environment where the external environment factors such as temperature, pressure, volume will not have affect the concentration of species that are participating in a chemical reaction. This is because there is no interaction between the substances in the system with matter in the surrounding.
An example of a closed system is a sealed bag of coffee bean.
It is important to note that for BOTH systems, it is possible for the exchange of heat between the system and surroundings.
Marking criteria:
1 mark = definition of open system
1 mark = definition of closed system
1 mark = an example of open system
1 mark = an example of closed system
Solution to Question 9:
Enthalpy refers to the total internal energy of system. Thus, if the kinetic energy of the species in a system is increased, the system’s enthalpy would increase. This can be done by increasing the system’s temperature.
The sign of a system’s enthalpy is important. This is because a negative change in enthalpy would indicate that the reaction is exothermic. Vice versa, if the sign of the change in enthalpy is positive, it would mean the reaction is endothermic.
Understanding whether a reaction is endothermic or exothermic will allow chemists to drive an equilibrium reaction in a desired direction. For equilibrium reactions, heat can be absorbed or inputted into the system to drive the equilibrium towards a preferred direction. You will learn how next week by understanding Le Chatelier’s Principle.
OR/
Understanding whether a reaction is endothermic or exothermic will allow chemists to determine whether they exploit the heat energy involved in the chemical reaction. For instance, exothermic reactions would give off heat into the surrounding environment. Chemists can make use of this heat energy to power other activities.
Marking criteria:
1 mark = Define enthalpy
2 mark = Describe the relationship between enthalpy with endothermic and exothermic reaction
1 mark = Outline the importance of understanding whether a reaction is exothermic or endothermic
Curveball Question Solutions
Solution to Curveball Question 1:
Steady state refers to a system where the change in concentration of the system’s species is constant due to the net force acting on the system being zero.
By definition, steady state can exist for static and dynamic equilibrium when it reaches equilibrium as, at equilibrium, the net force acting on the system is zero.
On top of static and dynamic equilibrium, you can also see steady state existing in a one-way ‘equilibrium reaction’ where the net force acting on the system is zero. It is one-way because the products does not react together to produce the reactants.
You will typically see this in an open system where both the change in concentration of the reactants and products can be constant. This is made apparent in a steady state gas equilibrium where the gas products is allowed escape from the open system, however, the change in the gas’s concentration is constant as more reactants are continuously supplied into the system to produce the product gases as the same amount of gas leaves the system.
Similarly, the same amount of reactants are being added into the system that are consumed at the same time to make the same amount of products. Although the change in the concentration is constant for both the reactants and product gases, the products are not reacting to form the reactants. Hence, one way ‘equilibrium’. This only occurs when no net force is acting on the system.
A typical example of a steady state is a bunsen burner burning at a constant blue flame. There is no apparent change in appearance as no net force is applied to the system. As gas of the flame evaporates into the atmosphere, more gas is supplied at a constant rate to maintain the flame. The gas that is expended will not come back into the system (one-way).
Marking criteria:
1 mark = Define steady state
1 mark = Explain steady state
1 mark = Example of steady state
Solution to Curveball Question 2:
Constant change in concentration means that the concentration of the species will increase and decrease by the same amount over time depending on the direction which the equilibrium reaction proceeds.
For example, for a chemical reaction such as 3A + 5B <-> 2C + 6D, if 5 moles of substance B is reacted to form 2 moles of C and 6 moles of D, there will always be a decrease of 3 moles of A.
Vice versa, there will always be 3 moles of A formed if 2 moles of C and 6 moles of D are consumed (reacted).
The change in [A] is always constant (or consistent to mole ratio) in both the forward and reverse reaction.
Equal change in concentration refers to the relative increase or decrease in concentration between species. For example, in the chemical reaction 2A + 2B <-> 2C + 5D, whenever 2 moles of A is reacted to 5 moles of D, A and B will always be decreased by 2 moles (we mentioned this earlier due to constant change in concentration). However, here in the second chemical equation, the change in concentration for both A and B are constant AND EQUAL. A and B both decreased by the same amount of moles.
Marking criteria:
2 mark = define constant and equal change in concentration
1 mark = explain the effect of constant change in concentration
1 mark = explain the effect of equal change in concentration
2 mark = use more than one chemical equation to illustrate the difference between constant and equal change in concentration
Solution to Curveball Question 3:
Surface area of a substance is the available space for reaction with another species. The rate of reaction relates to the rate at which reacting species are being converted into products for a given period of time. Substances that have bulky groups that are ‘shielding’ the important atoms that are required for a desired chemical reaction to take place would have a smaller surface area than substances that are linear (no bulky groups). This is because more particles are able to collide with the necessary atoms in substances that are linear. Therefore, increasing the surface area of reactants would increase the rate of reaction. Vice versa, decreasing the surface of reactants would decrease the rate of reaction.
Marking criteria:
1 mark = Define surface area
1 mark = Define rate of reaction
1 mark = Effect of larger surface area on rate of reaction
1 mark = Effect of smaller surface area on rate of reaction
Solution to Curveball Question 4:
Entropy refers to the number of ways or states that energy can be distributed to the species (particles) in the system.
It is NOT possible to have negative entropy in a system. However, it is possible to have negative CHANGE in system’s entropy and positive total change in entropy. This can be expressed in the following formula:
ΔS (total) = ΔS (system) + ΔS (surroundings)
If the change in entropy of the surrounding is greater than the change in entropy of the system, the total change in entropy is greater than zero.
Marking criteria:
1 mark = Define entropy
1 mark = Formula
1 mark = Explain scenario where there is a positive total change in entropy when change in system entropy is negative.
Solution to Curveball Question 5:
Temperature is a measure of average kinetic energy in species, typically expressed in kelvins or degrees celsius. Kinetic energy is the energy that such species possess and its magnitude relates to the speed at which the species move through a medium.
A system with a higher temperature will contain species with higher average kinetic energy.
Marking criteria:
1 mark = Define temperature
1 mark = Define kinetic energy
1 mark = Relationship between temperature and kinetic energy