HSC Chemistry Syllabus Notes
Module 7 / Inquiry Question 4
Learning Objective #1 – Investigate the structural formulae, properties and functional group including:
Primary alcohols
Secondary alcohols
Tertiary alcohols
Learning Objective #2 – Explain the properties within and between homologous series of alcohols with reference to intermolecular and intramolecular bonding present.
Learning Objective #3 – Conduct a practical investigation to measure and reliabily compare the enthalpy of combustion for a range of alcohols.
Learning Objective #4 – Write equations, state conditions and predict products to represent the reaction of alcohols, including but not limited to:
Combustion
Dehydration
Substitution with HX
Oxidation
Learning Objective #5 – Investigate the production of alcohols, including:
Substitution reactions of halogenated organic compounds
Fermentation
Learning Objective #6 – Investigate the products of the oxidation of primary and secondary alcohols.
Learning Objective #7 – Compare and contrast fuels from organic sources to biofuels, including ethanol.
NEW HSC Chemistry Syllabus Video – Alcohols
Week 11 Homework Questions
Week 11 Curveball Questions
Week 11 Extension Questions
Solutions to Week 11 Questions
Overview of Week 11 Inquiry Question
Welcome back to Week 11 of your HSC Chemistry Syllabus Notes!
In this week’s notes, we will be examining alcohols as a functional group specifically.
In the first learning objective, we will be exploring the three types of alcohols functional group including primary, secondary and tertiary alcohols in terms of their structural differences alongside any differing physical & chemical properties.
Next, we will explore how each of the three classes of alcohol functional groups undergo reactions such as combustion, dehydration, substitution with hydrogen halide (HX) and oxidation differently.
After that, we will explore the process of fermentation used to produce ethanol as a renewable source of biofuel.
Lastly, we will wrap up this week’s notes with a comparison in terms of advantages and disadvantage between the use of non-renewable and renewable sources of fuel.
Learning Objective #1 - Investigate the structural formulae, properties and functional group including:
- Primary Alcohols
- Secondary Alcohols
- Tertiary Alcohols
We have already talked about the physical shared by all alcohols in general in last week’s notes, except for the boiling points between primary, secondary and tertiary alcohols which we will explore in this week’s note.
So please revisit last week’s notes, if you need to revise on the other physical properties of alcohols homologues.
That being said, we will explain some differences in the chemical properties between primary, secondary and tertiary alcohols towards the end of this learning objective as well as in Learning Objective #4 and #6 which we haven’t explored in last week’s notes.
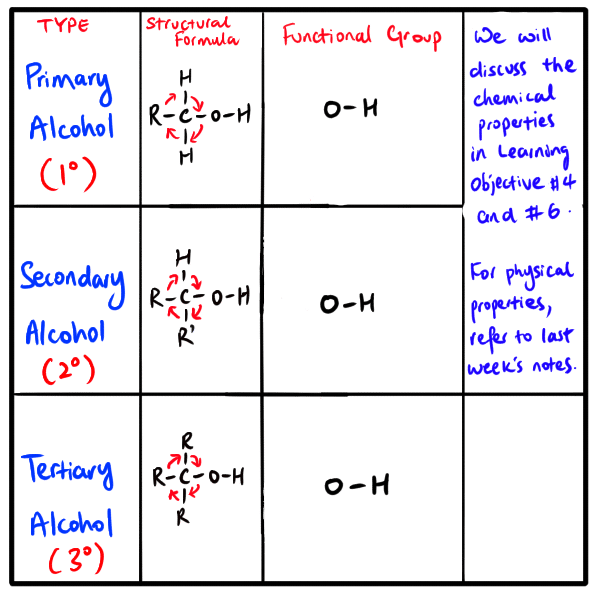
Anyways, returning to this learning objective. This question we want to address is what are the differences between primary, secondary and tertiary alcohols?
Well, primary alcohols are alcohols whereby the carbon atom that is bonded to the hydroxyl group (OH) is only bonded to one R group.
On the other hand, secondary alcohols are organic molecules whereby the two R groups (can same or different) are attached the carbon atom that is bonded to the hydroxyl group.
Lastly, in the case of tertiary alcohols, they have three R groups (can be same or different) are bonded to the carbon atom which is also bonded to the hydroxyl group.
NOTE: The ‘R’ groups used here to differentiate primary, secondary and tertiary molecules are groups that contain at least one carbon atom attached to hydrogen atoms. The R groups here cannot be hydrogen atoms.
Check out the following diagram that compares the differences between the three categories of alcohols.
NOTE: The direction in which you draw the R-C bond(s) does not matter. As you can see in the diagram, the red arrows are drawn to remind and show you that the chemical bonds can be rotated (clockwise or anti-clockwise)
NOTE: Some sources include methanol as a primary alcohol. Some do not. It is up to you to decide. It is probably more accurate to exclude it from the primary alcohol class.
So let’s explore in terms of melting & boiling point shared between primary, secondary and tertiary alcohol with similar molecular masses (i.e. same number of carbon atoms).
So, in the order of increasing melting & boiling points: Tertiary, secondary, primary alcohols and finally methanol.
An important factor that is contributing towards this trend is that an alkyl group is bigger in size than a hydrogen atom. But let’s explore why this is important in dictating the trend above.
As tertiary alcohol has more alkyl groups than secondary alcohols which has more alkyl groups than primary, the hydroxyl group is less exposed due to more alkyl groups. This hinders the extent at which hydrogen bonding can occur. This means that there would be less hydrogen bonding occurring in tertiary alcohol compared to secondary alcohols due to the hinderance of alkyl groups.
Therefore, tertiary alcohols have lower melting & boiling point than secondary alcohols and also the reason for why secondary alcohols have lower M.P and B.P than primary alcohols.
Needlessly, to say by now, methanol does not have any alkyl groups, hence, it has higher melting and boiling point than corresponding primary, secondary and tertiary alcohols.
As promised at the start of this learning objective, we will discuss about a difference in chemical property between primary, secondary and tertiary alcohols here.
This difference in chemical property amongst the three different types of alcohol is their acidity. In order of increasing acidity (from low to high): tertiary alcohol -> secondary alcohol -> primary alcohol -> methanol
For HSC Chemistry purposes, we can explain this trend by understanding that alkyl groups are better electron donators than hydrogen atoms.
Therefore, as the number of alkyl groups increases from primary to tertiary alcohol, more electron density is pushed towards the central carbon atom which passes on some of these electron towards the oxygen atom in the hydroxyl group. As a result, the electrons that oxygen attracts from hydrogen atom in the OH group is less extensive. Therefore, the energy required to break the OH bond increases. This makes it harder to break the hydroxyl bond and release a H+ ion.
Another way to explain this is that as the number of electron donating alkyl groups increases, the more unstable the conjugate base will be (due to increased number of electrons which the conjugate base carries) after the alcohol donates its hydrogen atom. Therefore, tertiary alcohols form the least stable conjugate base, thus, making it the weakest acid of the three.
The more detailed explanation of differing acidity lies in the hyperconjugation effect which we will not explore as it is outside the scope of the HSC Chemistry syllabus.
Lastly, in terms of chemical properties differences, due to their different number of alkyl groups present in the different types of alcohols, primary alcohol be oxidised to aldehyde and carboxylic acid but not to ketone.
In the case secondary alcohols, they can be oxidised to ketones but not to aldehyde or carboxylic acid (unless in extreme oxidative conditions).
Comparatively,, tertiary alcohol does not readily oxidise unless in extreme oxidative conditions involving the breaking of C-C bonds such as in combustion.
We will explore more about these different oxidation pathways of primary, secondary and tertiary alcohols in Learning Objective #6.
So, we just said that tertiary does not readily oxidise, i.e. does not really undergo oxidation reactions.
In Learning Objective #4, you will learn that for non-oxidation reactions (e.g. substitution with HX and hydration/dehydration of alcohol to form alkenes), tertiary alcohol will be more reactive
than secondary followed by primary and lastly methanol.
That is, the trend for different categories of alcohols undergoing oxidation reaction is opposite for the case of non-oxidation reactions.
Learning Objective #2 - Explain the properties within and between homologous series of alcohols with reference to intermolecular and intramolecular bonding present.
We have already examined the intermolecular and intramolecular bonding present in alcohol homologues in last week’s notes. So, for that, we will not repeat it here.
Feel re-visit last week’s notes for a recap.
Learning Objective #3 - Conduct a practical investigation to measure and reliably compare the enthalpy of combustion for a range of alcohols
Step 1: Measure 50mL of water using a 100mL measuring cylinder and transfer the water into a metal (copper or aluminium) calorimeter. Record the initial temperature of the water using a thermometer and leave the thermometer in the calorimeter.
Step 2: Use clamps and a retort stand to position the centre of the calorimeter’s base to be slightly on top of the spirit burner’s wick.
Step 3: Weigh the initial mass of the spirit burner containing methanol.
Step 4: Remove the recap of the burner and light the wick of the spirit burner and position the burner underneath the centre of the calorimeter’s base, minimising the distance between the burning wick and the base of the calorimeter.
Step 5: Slowly stir the water using the thermometer as the alcohol burns throughout the experiment.
Step 6: Put on the cap of the spirit burner to stop the ignition when the temperature shown on the thermometer is approximately 35 degrees celsius and record final temperature.
Step 7: Weigh the final mass of the spirit burner to determine the mass of methanol used.
Step 8: Repeat step 1 – 7 using a different spirit burner containing ethanol, propan-1-ol and butan-1-ol each time while using a fresh 50mL of water per experiment.
Step 9: Calculate the enthalpy of combustion for each of the four alcohols.
*Note you can calculate enthalpy of combustion (ΔHcombustion) using the m*c*ΔT formula. Furthermore, to calculate molar enthalpy of combustion of an alcohol, you just simply divide it by the moles of the particular alcohol used.
As per usual, the mass (m) here will be solution heated in the calorimeter which, in this experiment, it will be the mass of water.
The c for this experiment is the specific heat capacity of water (However, you may be given the specific heat capacity of the calorimeter. If so, you should use the formula, ΔH = [ (msolution x csolution) + (ccalorimeter) ] x ΔT, which is a formula that we have derived and explained in Module 6’s Notes under enthalpy of neutralisation section. Please revisit the Module 6’s notes if you need to revise on the formula.
The ΔT for this experiment is the change in temperature of the water: Final temperature – Initial temperature.
Improving reliability of results
A draught shield can be used to prevent cool air in the room from carrying heat away from the calorimeter to make the results more reliable (closeness of results) and some of the heat radiating from the calorimeter can be re-directed back to the calorimeter from the draught shield.
You should also repeat the experiment for each alcohol to test for the reliability of your results. As a class, it is likely that you will combining results rather than doing it multiple times per group which is fine. Although you can argue that there may be some validity issues surrounding this on how your cumulative results are derived from different people performing the experiment.
You can add a lid to the calorimeter if you did not have one in order to minimise the heat loss to the surrounding, i.e. environment.
Learning Objective #4 - Write equations, state conditions and predict products to represent the reaction of alcohol, including but not limited to:
- Combustion
- Dehydration
- Substitution with HX
- Oxidation
Combustion of alcohols
Primary, secondary and tertiary alcohols can be readily combust in the presence of oxygen to form carbon dioxide and water as they’re flammable with a low ignition temperature.
Combustion is a form of extreme oxidation reaction where all chemical bonds of the alcohol and oxygen are broken so that all three forms of alcohols readily combust.
However, as we will explore later in Learning Objective #6, in normal oxidation reactions where common oxidising agents are used, only primary and secondary alcohols are oxidised.
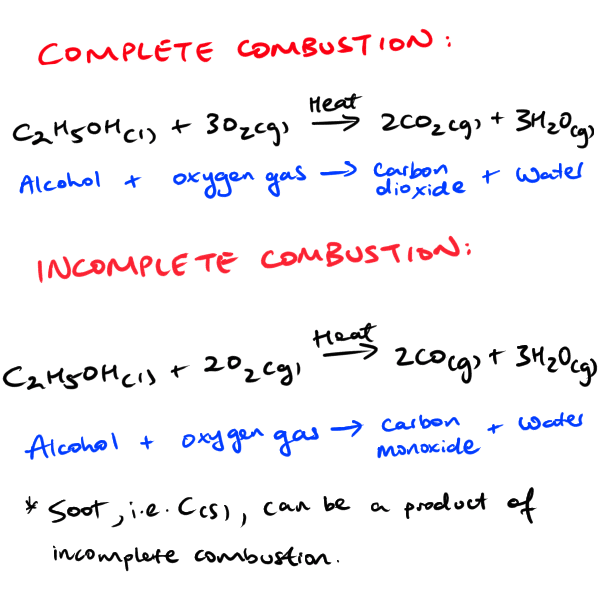
There are two forms of combustion which we have explored in Preliminary HSC Chemistry. These are complete and incomplete combustion.
In completion combustion, both carbon dioxide and water are formed as products due to excess oxygen being available. In contrast, during incomplete combustion, other products such as carbon monoxide and soot (solid, black-coloured carbon particles) are be produced due to limited oxygen as a reactant.
SIDE NOTE: Carbon dioxide can still be found in incomplete reactions. However, you will also see either CO(g) or C(s) or both formed alongside it. Sometimes, you don’t see carbon dioxide in incomplete reactions at all. It really depends if the carbon in your reactant (e.g. alcohol) is oxidised to carbon dioxide. But with limited oxygen, thus resulting in incomplete combustion, you will definitely see C(s) or CO(g).
Reaction conditions: Heating the alcohol to its ignition temperature.
Dehydration of alcohols
Reaction condition: Heating the alcohol in the presence of a concentrated acid catalyst (e.g. H2SO4 or H3PO4) at 170 degrees celsius. The product as a result of the dehydration of alcohol is an alkene as well as water as a byproduct.
Since alkene is formed as a product, the dehydration of alcohol can only occur if the alcohol has two or more carbon atoms.
NOTE: If the temperature is not suffice then ethers would be produced rather than alkenes. This is a condensation reaction as water is released as a by-product and the product is larger than the reactants, i.e. alcohol. Dehydration is a type of condensation reaction.
We will explore more about condensation reactions in Week 13’s Notes when we explore polymers.
Substitution reaction involving alcohols with HX (hydrogen halide)
What happens in substitution reaction is that the HX ‘attack’s (interacts) and react with the C – OH bond to free the hydroxyl group and substitute the original alcohol molecule with the halogen atom “X”.
The free, negatively charged hydroxide ion can bond with the positively charged hydrogen ion (from the HX molecule) to form water as a by-product.
If we wish to determine the alcohol is primary, secondary or tertiary, we can use the Lucas Test which involves the use of a Lucas’ reagent which is essentially a solution comprised of an anhydrous zinc chloride catalyst dissolved in concentrated hydrochloric acid solution.
The products produced is a chloroalkane (R-Cl) and water. To determine whether the alcohol is primary, secondary or tertiary, the rate of reaction can be used.
Tertiary alcohol is able to completely react with the hydrochloric acid via substitution reaction in less than one minute to form the cloudy, white haloalkane precipitate and water as a by-product.
This means that two layers are formed (originally there is only one layer of alcohol solution at start of experiment). The bottom layer is the precipitate that is more dense and the top layer is the water alongside any excess alcohol and the zinc chloride catalyst.
For the case of secondary alcohol, the reaction typically requires 2-5 minutes. Again, the products are precipitate haloalkane and water.
Lastly, it would take more than 6 minutes for primary alcohol to react with the hydrochloric acid in the case of primary alcohols. When the reaction is complete, the haloalkane product is formed as a precipitate as well as water.
You may need to heat the solution containing primary alcohol and hydrogen halide (HX) to speed up the rate of reaction.
Recall that in learning objective #1, we mentioned that for non-oxidation reactions, tertiary alcohols are more reactive than secondary and primary alcohols. Substitution reactions are is example of this.
Oxidation of alcohols
We will explore the equations, conditions and products for the oxidation of alcohols in Learning objective #6.
Learning Objective #5 - Investigate the production of alcohols, including:
- Substitution reactions of halogenated organic compounds
- Fermentation
Substitution reaction of halogenated organic compounds to produce alcohol
It is possible to dissolve an alkyl halide in water such that a substitution reaction can occur to produce an alcohol as a product and a hydrogen halide (H-X) molecule.
Similar to other substitution reactions that we have explored, this involves the water interacting and reacting with the R-X bond in the alkyl halide so that the hydroxyl group (-OH) bonds and substitutes the halogen atom from the alkyl halide, forming the R-OH alcohol molecule.
The halogen atom, X-, that is substituted out of the alkyl halide can bond with the remaining hydrogen atom from the water molecule to form a hydrogen halide (HX) molecule.
As we have explored in the Lucas Test in the previous learning objective, similar to how tertiary alcohols are most reactive followed by secondary then primary alcohols, it also applied here.
That is, in decreasing order of reactivity for substitution (non-oxidation) reactions, tertiary alkyl halides undergoes the substitution reaction faster than secondary and, finally, primary alkyl halides.
NOTE: Alternatively, it is possible to convert alkyl halides into alcohol by dissolving it in an aqueous solution of a strong Group 1 base such as NaOH, KOH or C2OH.
Fermentation to produce alcohol
Fermentation is an exothermic reaction involving the conversion of glucose into ethanol and carbon dioxide through the use of enzymes produced by microbes.
Conditions required for fermentation reaction:
Yeast (containing the enzyme, zymase, to break down glucose to ethanol)
Anaerobic reaction conditions (i.e. absence of oxygen). This is because if oxygen is present, the enzymes will convert or oxidise the glucose into carbon dioxide and water rather than ethanol.
Reaction temperature of approximately 37 degrees celsius. Lower temperatures will slow down the process of fermentation, i.e. takes longer time.
Aqueous solution of glucose as a reactant to convert into ethanol (and carbon dioxide)
Simplified Procedure:
Step 1: Dissolve 25 grams of glucose in 100mL of water inside a 500mL conical flask.
Step 2: Add 5 grams of yeast to the solution and put a stopper on the conical flask.
Step 3: Store the conical flask in an environment from approximately equal to normal room temperature, i.e ~25 degrees celsius to 37 degrees celsius (optimal for highest rate of reaction).
Step 4: Due to the ethanol’s solubility in water, caused by the hydrogen bonding between alcohol and water, it is difficult to obtain 100% pure ethanol. However, fractional distillation can be used to obtain quite pure ethanol (95% v/v)
NOTE: Yeast will die once the alcohol concentration in the fermentation vessel reaches approximately 15% (v/v), recently this percentage has increased to 17% by introducing experimenting with yeasts with greater alcohol tolerance. Fractional distillation is required to purify the ethanol product, for example, to 95% v/v.
NOTE: Since fermentation is an exothermic reaction, the heat that is evolving from the reaction must escape the system so that a temperature of 37 degrees celsius can be maintained to keep prevent enzymes from denaturing. If all enzymes are denatured, the reaction cannot be catalysed and stops the fermentation.
NOTE: If sucrose or starch is the starting product, then the sucrose needs to undergo a hydrolysis reaction by reacting with water (in the presence of enzymes) to form glucose. The glucose can then be fermented to produce ethanol and carbon dioxide. This is what happens in industry where polysaccharide (e.g. starch) are broken into monosaccharides (e.g glucose) before fermentation happens.
Hydration of alkene to produce alcohol
We have already mentioned in the previous learning objective that the dehydration of alcohol to produce alkene is reversible.
That is, alkenes can be hydrated to produce alcohol through the reaction with water (i.e. hydration reaction) in the presence of a dilute acid such as dilute sulfuric acid.
Learning Objective #6 - Investigate the products of the oxidation of primary and secondary alcohols
Here, we are going to examine how we can react primary and secondary alcohols with reagents (substance to initiate a reaction) to transform them into different products.
Depending on the oxidising conditions, primary alcohols can either be converted (or oxidised) into an aldehyde or carboxylic acid. Comparatively, secondary alcohols can be oxidised to produce ketones.
For HSC purpose, we will not be exploring the oxidisation of tertiary alcohols as you can assume that it is NOT possible to oxidise tertiary alcohols. This is because there is no hydrogen atom attached to the carbon atom that is bonded to the OH group.
Note that: In practice, it is possible to oxidise tertiary alcohol in practice by breaking carbon-carbon bonds to form the C=O bond although much stronger acids and higher temperatures would be required such as in combustion. However, the oxidation of tertiary alcohols is not useful in synthetic chemistry as we will lose an alkyl (R) group in the product as we need to break the carbon-carbon bond (or also known as the R-C bond). This will therefore disrupting the parent carbon chain structure which we generally do not want in synthesis.
When an alcohol is oxidised, the oxidation number of the central carbon attached to the oxygen of the alcohol increases with the number of chemical bonds. So, a single bond C-O has a lower oxidation state than a C=O bond.
Example on the change in oxidation state from primary alcohol to aldehyde
This is where redox reactions that you learnt in the Preliminary HSC Course comes back into play.
So, the above oxidation reaction is reversible, where the reverse reaction will be the reduction of aldehyde to re-form the primary alcohol. Why is it called a reduction reaction?
Well, a reduction involves a decrease in oxidation state and vice verse for oxidation reactions.
To convert an aldehyde into an alcohol in the above example, there will the a decrease in the oxidation of the central carbon atom in the aldehyde (methanal) of 0 to an oxidation state of -2 in the (methanol) product. Hence, it is called a reduction reaction.
Oxidation of primary alcohols
To oxidise primary alcohol to create an aldehyde, we can use the oxidising reagent pyridinium chlorochromate (PCC) that is dissolved in CH2Cl2. Like all oxidsing reagents, they are reduced after the redox reaction as we have learnt in HSC Preliminary Chemistry.
For instance, in this case, the chromate’s oxidation state in PCC is lowered (hence reduced) and the carbon atom attached to the oxygen atom in the primary alcohol is oxidised as it is being transformed into an aldehyde.
This means that the carbon atom attached to the oxygen atom (C=O) in aldehyde product has a higher oxidisation state than the carbon attached to the oxygen (C-O) in the primary alcohol. This was seen in the previous diagram where we compared the oxidation state of the central carbon atom attached to the oxygen in alcohol and aldehyde.
The technique used to convert primary alcohols into aldehyde is can be done using simple distillation where aldehyde is formed as the distillate.
Oxidation of Primary Alcohol to Carboxylic Acid
However, if a stronger oxidising reagent is used, such as acidified sodium chromate, acidified potassium chromate or acidified chromium trioxide, the aldehyde product from the primary alcohol will be furthered oxidise to form a carboxylic acid.
The technique used here to convert primary alcohols into carboxylic acids is heating under reflux to allow the reaction to proceed for a longer period of time so that the aldehyde can be further oxidised into a carboxylic acid.
We can acidify the three solutions named above by adding sulfuric or acetic acid solutions to them.
It should be noted that even if PCC reagent is used to oxidise primary alcohol to aldehyde, the aldehyde product will still readily oxidises to form a carboxylic acid IF exposed to oxygen in the air despite not adding any strong oxidising agents.
Oxidation of secondary alcohols
Similarly to the oxidation of primary alcohol, the secondary alcohol has a hydrogen atom removed from the carbon atom (from C-H bond) and a hydrogen removed from the oxygen atom (from O-H bond).
So, the product is that the carbon atom having two chemical bonds to the oxygen atom (C=O) in the ketone compared to only one chemical bond (C-O) in the secondary alcohol. This means that the carbon atom is oxidised in the secondary alcohol to form ketone, resulting in the central carbon atom has a higher oxidation state in the ketone.
NOTE: You can calculate the oxidation state of the central carbon atom in ketone and compare it to the oxidation state of the central carbon atom in the secondary primary alcohol, similar to the previous case between which we did for primary alcohol & aldehyde.
In terms of the reagent that we can use to transform (oxidise) secondary alcohols into ketones, we can use acidified sodium dichromate or acidified potassium dichromate or acidified chromium trioxide solutions. We can acidify such solutions by which is adding sulfuric or acetic acid solutions to them.
NOTE: For HSC Chemistry purposes, it is ketones do not oxidise into carboxylic acids as it does not have a hydrogen atom that is attached to a carbon atom which is double bonded an oxygen atom.
In practice, it is possible to further oxidise ketones into carboxylic acids (e.g. combustion) however it is not useful as the parent carbon chain structure of the molecule will be disrupted as the C-R bond will be broken.
Learning Objective #7 Compare and contrast fuels from organic sources and biofuels, including ethanol
Non-renewable fuels vs Renewable fuels
As we have discussed in Week 9’s Notes, fossil fuel such as petroleum, coal and natural gas, are non-renewable sources but are currently our main organic sources of fuel.
Comparatively, biofuels such as ethanol are derived from living matter and they’re often deemed renewable sources as they can be grown or produced at a large scale in a short time.
If you recall from Preliminary HSC Chemistry, it is possible to define a substance as ‘renewable’ if the rate of production is able to exceed the rate of consumption.
Comparison between fossil fuel and biofuels
Advantages of fossil fuels:
The use of fossil fuels in vehicles (mainly octane) saves time used in refueling as octane has a higher energy output per gram (and thus per mole) compared to ethanol, thus can travel a longer distance before the need for re-fuelling. The values are 46 kJ/g and 20.6 kJ/g respectively.
Plus all the disadvantages of biofuels – see in biofuel disadvantages section below!
Disadvantages of fossil fuels:
As the formation of fossil fuels takes millions of years, they are considered non-renewable. Therefore, it is not sustainable in the long term to meet the growing world population’s demand for energy usage.
Furthermore, as quantity of fossil fuel decreases, the price of fossil fuels will rise which will become less affordable to consumers.
As the price of fossil fuel increases with declining quantity, the synthetic polymers used in everyday life such as low-density polyethylene used to make plastic bags produced from monomers derived from fossil fuels such as petroleum would therefore also increase.
NOTE: We will learn more about polymers in Week 13’s Notes.
Fossil fuels used in cars (majority octane) burn less cleanly than biofuels such as ethanol as it requires more oxygen for one mole of octane to undergo complete combustion. This would mean that toxic carbon monoxide will be produced as a product which can harmful to living organisms such as humans.
Advantages of biofuels:
As crops can be grown at a large scale and broken down into glucose which can be subsequently fermented to produce ethanol, it is renewable. Therefore, it has the potential to sustain the growing world population’s demand for energy usage in the long term
Biofuels like ethanol burns more cleanly (complete combustion) than from fossil fuels like octane as less oxygen is required per mole of ethanol as shown in the equations below:
C2H5OH(l) + 7/2 O2(g) -> 3H2O(g) + 2CO2(g)
C8H18(l) + 25/2 O2(g) -> 9H2O(g) + 8CO2(g)
There are current research on developing the use of photobioreactors in producing biodisel from microalgae at a large scale. Microalgae provides many advantages over current plants that are currently used to produce biofuel (such as sugar cane to produce ethanol). This includes the fact that algae does not require to be grown on agricultural land so the arable land can be used to grow food for growing world population. Also, microalgae can grow during any time of the year as it can sustain harsh ambient conditions, reducing water and pesticides required (lowering cost) and higher yield per unit area (from 10 to 100 times more than conventional biofuel such as ethanol).
If biofuels are used by cars, factories, tractors to harvest and transport crops for processing, then the will be a greater net reduction in carbon dioxide greenhouse gas as seen in the formula above. Also, the plants grown to produce glucose will perform photosynthesis to consume carbon dioxide, which lowers the total carbon dioxide produced from combustion.
Disadvantages of biofuels:
The use of large amounts of land to grow crops for the purpose of fuel production reduces the land available for food production. With the increasing world population, both the demand for higher food and energy consumption (e.g. energy provided by biofuels) are both increasing and they both need to be both rather than just energy. Due to the limited land available on Earth, different food and biofuel production processes would be required.
The energy used by tractors to harvest the crops that would be used to produce ethanol is currently supplied by the burning of fossil fuels.
The energy used for the fermentation to produce alcohol and distillation to increase purity of alcohol currently derived from the burning fossil fuels.
The energy used by trucks to transport crops to to biofuel manufacturing plants and the ethanol product to relevant stores to sell to consumers are currently derived from the burning of fossil fuels.
Due to limited amount of land on Earth, the landfills are already burdened with the large amount of rubbish. There will be problems in the future in storing large volume of fermentation waste if a switch from fossil fuels to completely renewable biofuel production via fermentation in the future.
The limitations currently is that the cultivation cost of microalgae is greater than plants such as sugar cane in producing biofuel. Furthermore, there will be problem with producing microalgae arises when the availability of sunlight varies. Perhaps, with recombinant DNA technology (a form of biotechnology), a gene can be identified and inserted into microalgae that enhances its yield production during low sunlight conditions.
Not all vehicles’ engines are currently compatible to use 100% ethanol and so some car engines may need to be modified to use 100% ethanol.
Week 11 Homework Questions
Week 11 Homework Question #1: In your HSC Chemistry course, you performed an experiment to determine the enthalpy of combustion for a range of alcohols. Identify three potential risks involved in the experiment and the safety precautions that you should take to minimise or prevent such risks.
Week 11 Homework Question #2: For the same experiment as outlined in the above question, explain how you can improve the accuracy of the results? Furthermore, what are the control, independent variable, dependent variable and controlled variables of the experiment?
Week 11 Homework Question #3:
Week 11 Curveball Questions
Insert calculation questions of the oxidation state of atoms in primary and secondary alcohol, ketone, aldehyde and carboxylic acids.