HSC Chemistry Syllabus Notes -
Module 7 / Inquiry Question 1
Overview of Week 8’s Inquiry Question – How do we systematically name organic chemical compounds?
Learning Objective #1 – Investigate the nomenclature of organic chemicals, up to C8, using IUPAC conventions, including simple methyl and ethyl branched chains, including:
alkanes
alkenes
alkynes
alcohols (primary, secondary and tertiary)
aldehydes and ketones
carboxylic acids
amines and amides
halogenated organic compounds
Learning Objective #2 – Explore and distinguish the different types of structural isomers, including saturated and unsaturated hydrocarbons, including:
chain isomers
position isomers
functional group isomers
NEW HSC Chemistry Syllabus Video – Organic Chemistry Nomenclature
Week 8 Homework Set (Essential for Band 5!)
Week 8 Curveball Questions (Moving from Band 5 to Band 6)
Week 8 Extension Questions
Solutions to Week 8 Questions
Overview of Week 8's Inquiry Question
Welcome to Week 8 of your HSC Chemistry Syllabus Notes!
We will be diving into sort of a new area here involving organic molecules, although we have explored organic molecules before. These are essentially molecules that have carbon and hydrogen atoms in them. Perhaps one that you may recognise from Module 6 is acetic acid, i.e. CH3COOH.
In this week’s notes we will explore how you can name a variety of organic compounds belonging to different groups which we call homologous series (we will explore them more in depth in next week’s notes).
The naming system we will be following is the IUPAC nomenclature methodology which is what NESA wants us to use.
During our journey of learning the basics of organic chemistry nomenclature, we will also explore different substituent groups and functional groups which should be fairly interesting too.
At the end of this week’s notes, we will close off by exploring different types of structural isomers including chain isomers, position isomers and functional group isomers.
Learning Objective #1 - Investigate the IUPAC nomenclature for alkanes
In this learning objective, we will explore how we can name alkanes up with eight carbons using IUPAC rules. As you may expect, there are some different IUPAC rules for naming larger and more complex organic molecules and thus they not explored in HSC Chemistry as a foundation course for first year university chemistry.
Before we get started, let’s define some terms which we will be using:
Parent chain: The longest, continuous carbon chain in an organic molecule made of carbons and hydrogens.
Substituent: A side chain (or group) or atom that is attached to the parent carbon chain which replaced a hydrogen atom. A substituent group or atom does not necessarily need to have a carbon atom.
Example: A chlorine atom attached to the parent carbon chain can be a substituent.
Example: A methyl group (-CH3) attached to a carbon atom in the parent carbon chain can be a substituent.
Functional Group: A side chain or group that is attached to the parent carbon chain. It does not need to necessarily have a carbon atom. A functional group is a substituent but a substituent may not be a functional group. Whether a substituent is a functional group, it will depend on the nature of the chemical molecule. The main difference is that a functional group provides a distinctive chemical property for the molecule to undergo certain chemical reactions.
NOTE: Alkanes generally are very weak functional groups (and substituent) and, whether or not they are a functional group, it will depend on the molecule.
Naming Unbranched Alkanes from C1 to C8
Example: What is the name of the molecular with the molecular formula: C6H14?
Answer: Hexane
As you can see, naming unbranched alkanes are easy, i.e. alkanes that do not have any substituent groups and thus linear.
Simply just combine the prefix (depending on the number of carbon atom) and the suffix ‘ane’ and you got the name of the alkane hydrocarbon!
Naming alkanes with substituent group(s)
Now let’s examine the process to name alkanes that has substituent group(s)!
Step 1: Identify the parent chain to be the parent name of the molecule. That is, the longest, continuous carbon chain.
As you have explored in Preliminary HSC Chemistry, all alkanes end with the term ‘ane’. Therefore, you should have ‘ane‘ at the end of your alkane’s root-name.
NOTE: In the case where there are two equally longest chain carbons, you would choose the one that incorporates the most substituents.
Step 2: Starting from a side of the parent carbon chain, number the parent carbon chain (by assigning numbers to carbon atoms) on the basis that the first substituent has the smallest number. This means that we need to start numbering the parent carbon on the side where we will encounter the first substituent as soon as possible.
NOTE: Our goal here is to assign the smallest carbon number to every substituent groups as possible and the above rule of thumb in Step #2 generally does the job well.
You will see that later, there will be naming priorities for different functional groups. In that case, you will have to assign lowest possible number to the highest priority functional group. Then, assign the lowest carbon number possible to the compound’s other substituent groups. However, for alkanes, there is no functional group! So, we won’t worry about this here.
NOTE: If the substituents are located at the same carbon-atom distance from different sides of the parent chain, then we will need to examine the next, nearest substituent. The side from which we can assign the lowest number to the next, nearest substituent would be the direction in which we will number our carbons.
NOTE: If this rule fails, then you got a real curveball! Perhaps, all of your substituents are in the same carbon distance to each other OR perhaps you don’t have a third substituent (the more likely case). In this case, name from the side in which the substituent appears first on the alphabet (first letter). For example, suppose you only have two substituents branching off the parent chain, which are a methyl group (- CH3) and a propyl group (- CH2CH2CH3) with the same carbon distance from each side of the parent chain. Well, we then should start numbering the parent chain from the side that is closer to the methyl group as ‘m‘ appears before ‘p’ in the alphabet. Therefore, after numbering, the methyl group will be attached a carbon atom in the parent carbon chain with a lower assigned carbon number.
Step 3: Use prefix to indicate the number of identical substituent groups or atoms (only if there is two or more) attached to carbon atoms in the parent chain and add prefix to the substituent groups’ root name.
Prefixes from 2 – 8 are: di, tri, tetra, penta, hexa, hepta, sept and octa respectively.
Step 4: Use number to label substituent corresponding to carbon atom which it is attached to with an assigned carbon number as per Step 2.
Separate the number assigned to substituent with the substituent name using a dash “-” symbol.
For multiple same substituents groups or atoms that are attached carbon atom(s) in the parent chain, separate their assigned number using a comma “,” symbol.
Step 5: Arrange the substituents according to alphabetical order AND combining the parent carbon chain’s name at the end without a space.
Awesome! Now we have explored Step 1 – Step 5 that will be help us in naming alkanes with substituent group(s).
However, before we actually get into a practical example, we need to examine what are the substituent groups that we need to know how HSC Chemistry.
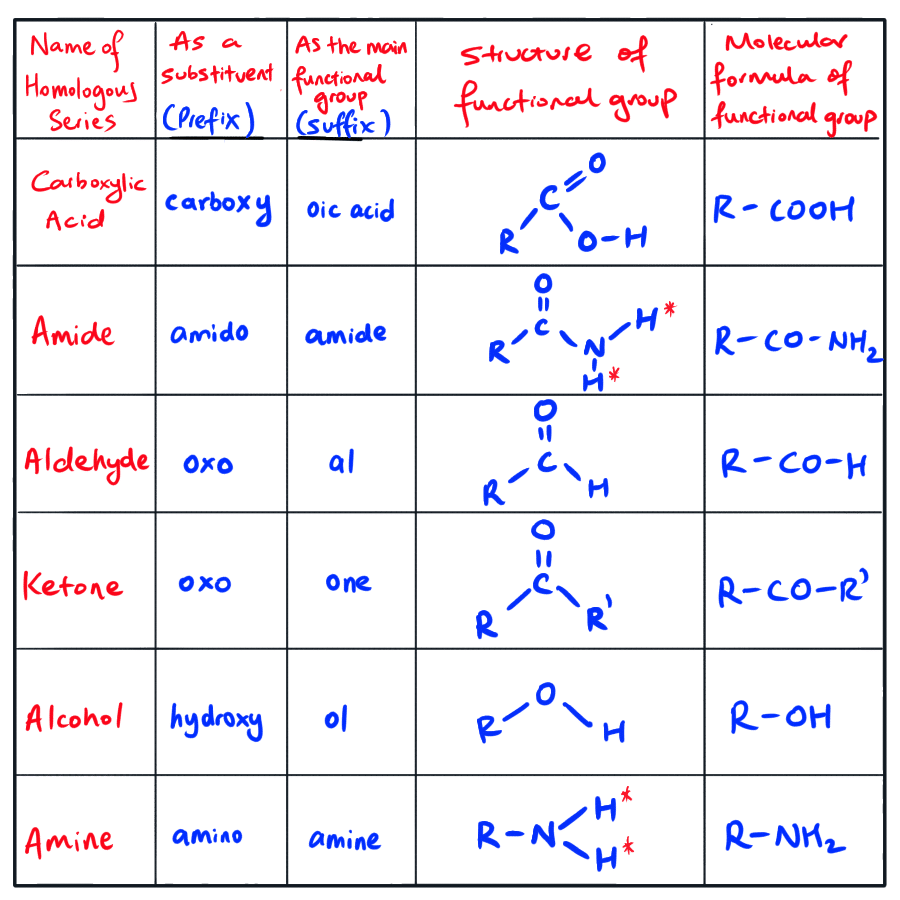
The following tables exhibit the substituents that you need to know how HSC Chemistry!
List of substituent and/or functional groups for HSC Chemistry
NOTE: The R, R’ and R” (R groups) can be the same group OR they can be different.
A R group can be hydrogen atom OR a group comprised of carbon atom(s) bonded hydrogen atom(s).
When R group is used to differentiate primary, secondary and tertiary versions of a functional group, the R group is strictly used to refer to a group containing at least one carbon atom attached to hydrogen atoms. We will formally introduce & explore about primary/secondary/tertiary versions in later weeks.
NOTE: One homologous series that’s NOT included in the table above is alkanes.
It is important to note that there is no real functional group in alkanes.
However, alkanes does have a suffix if the parent chain is consists of C-C bonds and the parent chain DOES NOT have any other higher priority functional (e.g. COOH) attached to it. The suffix for alkanes is ‘ane‘.
Similar to other homologous series, if alkanes exists as as a substituent, we don’t use its suffix. Instead, we use its prefix. The prefix for alkanes would be the name of the applicable alkyl group – e.g. methyl, ethyl, propyl, etc.
NOTE: The prefix for alkenes as a substituent group is the name of the applicable alkenyl group – e.g. ethenyl, propenyl, butenyl, etc.
NOTE: The prefix for alkynes as a substituent group is the name of the applicable alkynyl group, e.g. ethynyl, propynyl, butynyl, etc.
More on these hydrocarbon substituent groups and their structures in the table below.
Alkyl, Alkenyl and Alkynyl Substituent Groups
The naming of prefix for alkyl, alkenyl and alkynyl groups are pretty simple. It follows a simple formula.
We have already explored the appropriate prefix based on the number of carbons in the table above labelled “Naming unbranched Alkanes”.
E.g. Hydrocarbons (Alkanes or Alkenes or Alkynes) with one carbon has prefix “Meth”
E.g. Hydrocarbons with two carbons has prefix “Eth”, three carbons has prefix “Prop”, etc.
Prefix of alkyl substituent groups = Appropriate prefix based on number of carbon atoms + “yl”.
Prefix of alkenyl substituent groups = Appropriate prefix based on number of carbon atoms + “enyl”.
Yes, methylene is an exception as it doesn’t follow the same format of adding ‘enyl‘ after meth.
Prefix of alkynyl substituent groups = Appropriate prefix based on number of carbon atoms + “ynyl”.
NOTE: We can express the organic molecules’ molecular formula using stick structures as depicted using pink lines in diagram above.
We deliberately drew yellow dots on the line structure to depict how, at every yellow dot, there is a carbon atom. You should NOT draw the yellow dots in your line structure as we only did it here to get the idea through to you.
In line structures, we DO NOT show hydrogen atoms that are attached to the carbon atoms (yellow dots in diagram) as that’s the purpose of line structures to make it faster and easier to draw, read and communicate the chemical structure of organic molecules.
NOTE: In the table above, we have only shown C1 – C5 for alkyl, alkenyl and alkynyl substituent groups. We trust that you should now be able to recognise, name and draw C6 – C8 substituent groups by following the pattern depicted in the table.
If you have any trouble, feel free to let HSC mentors know on ConquerHSC’s discord community!
EXAMPLE: Naming branched alkanes (with substituent groups)
So, it’s finally time to do some practice examples on naming alkanes with substituent groups!
To do this, let’s follow steps 1 – 5 that we talked about at the beginning of Learning Objective #1 in this week’s notes as well as using what we learnt about substituent groups!
Learning Objective #1 - Investigate the IUPAC nomenclature for alkenes
Now let’s move onto naming alkenes! These are organic molecules with a carbon double bond, (C = C).
Basically, to name alkenes, we use the same procedure that we have outlined above for alkanes. However, there are few exceptions or things that you need take into consideration when naming alkene molecules.
These important exceptions are for alkenes are:
The parent carbon chain must contain the double-bond(s) (C=C) functional group.
Like all alkenes molecules, there is an ‘ene‘ after the root-name of the alkene molecule.
The C=C functional group has highest priority in alkene molecules. Therefore you should assign “1” to the the first carbon you encounter that makes up the C=C bond from one end of the parent carbon chain.
Example: But-2-ene, where the “2” indicates that the carbon double bond (C=C) is found on the second carbon atom in butene molecule. Notice that you include the position (“2”) of the carbon double bond (C=C) before you write the suffix for alkene “ene” and it separates using dash symbols “-” as per IUPAC conventions.
NOTE: Keep in mind that you must assign the least carbon number as possible to all of your substituent groups. So, check your work once you are finished!
There are special cases when you are doing with cyclic compounds but these exceptions NOT necessary HSC Chemistry. You will learn in Higher Chemistry 1A if you decide to take Chemistry university.
Learning Objective #1 - Investigate the IUPAC nomenclature for alkynes
Moving on to the next station, alkynes! These are organic molecules with a triple carbon bond, (C ≡ C).
To name alkynes, we use the same method that we used to name alkanes. However, again, there are some exceptions that you will need to evaluate.
The parent carbon chain must contain the triple bond(s) (C ≡ C) functional group.
Like all alkynes molecules, there is an ‘yne‘ after the root-name of the alkyne molecule.
The C≡C functional group has the highest priority in alkyne molecules. Therefore, you should assign “1” to the first carbon you encounter that makes up the C ≡ C bond from one end of the parent carbon chain.
In an organic molecule that contains both double and triple carbon bonds, they have equal priority. This means whichever of the two functional group appears on first from one side of the parent carbon chain, it will have the lower assigned carbon number.
As mentioned in the alkane section, if the double and triple carbon bonds are in at equal carbon atom distance from each side of the parent carbon chain, we use assign the lowest carbon number according to alphabetical order, the carbon attached to the double bond (-ene-) will be assigned a lower carbon number than the carbon atom attached to the triple bond (-yne-) as e comes before y in the alphabet.
NOTE: Keep in mind that you must assign the least carbon number as possible to all your substituent groups. So, check your work once you are finished!
Moving into alcohols, aldehydes, ketones, carboxylic acids, amine, amides & halogenated compounds
For alkanes, alkenes, alkynes and halogenated compounds, the substituent groups, i.e. alkyl, alkenyl, alkynyl and halogen groups, have equal priority and thus are named according to the alphabetical order of their substituent group’s prefix.
However, for the following compounds, the highest priority substituent (or functional group to be more specific) are based on the following functional group priority naming order:
Highest priority Side
Carboxylic acid group
Amide group
Aldehyde group
Ketone group
Alcohol group
Amine group
Lowest priority Side
Alkene (C=C) and alkynes (C≡C) have equal priority as we have mentioned before and they both have lower priority than amine groups. So, ‘en’ (double bond) and ‘yn’ (triple bond) should be named alphabetically if they’re treated as substituent groups.
This is, of course, followed by the following substituent groups have equal priority and thus are named in the order in an alphabetical order which we have mentioned:
Alkyl, Alkenyl, Alkynyl groups and halogen groups
NOTE: Halogens are not treated as functional group in organic chemistry nomenclature. However, when the question is not concerning nomenclature, halogen are treated as functional group as they give chemical properties to the organic molecule.
NOTE: There are many more other functional groups that fits between these groups but they are not relevant for HSC Chemistry purposes as they’re outside the scope of what the syllabus required us to know.
Learning Objective #1 - Investigate the IUPAC nomenclature for alcohols
A parent carbon chain with hydroxyl group as the highest priority substituent group is known as an alcohol.
To name an alcohol, we can use the following the steps:
Step 1: Identify the parent carbon chain, i.e. the longest, continuous carbon chain, that comprises of the highest priority functional group. In this case, the hydroxyl group (- OH) is the highest priority group.
Step 2: Assign carbon numbers to every atom in the parent carbon chain with the carbon atom that is bonded to the highest priority group where the highest priority functional group have the the lowest carbon number.
Step 3: Name any double and triple bonds in a similar fashion as we have discussed previously.
Step 4: Since the parent carbon chain has hydroxyl group as the highest priority group, it is an alcohol. The organic molecule that is an alcohol has the suffix ‘ol’. Therefore, we need to replace the ‘e‘ at the end of the root-name of the organic molecule with the relevant suffix. In this case of alcohols, the suffix after the root-name would be ‘ol’. Again, like other cases, there’s no space between root-name and suffix. Include the carbon number too before the suffix using dash symbols “-” to signal where the main functional group is located.
Finally, use the relevant prefix based on carbon number. E.g. Two carbons = ‘eth’. Three carbons = ‘Prop’.
Step 5: Name any other other substituent groups (disregarding priority order now) that are present in the organic molecule in alphabetical order, according to their prefix names, and assigning lowest carbon position number to every substituent group as possible.
EXAMPLE: Naming an alcohol without substituents
NOTE: Since alcohol is the main functional group (highest priority functional group), we use the suffix for alcohol ‘ol’ rather than its prefix ‘hydroxyl’.
We only use the prefix if alcohol is treated as a substituent. That is, it is not the highest priority functional group in the molecule.
Please refer back to the table that we explored earlier in this week’s notes that contains the prefix and suffix of each functional group if you have forgotten.
EXAMPLE: Naming an alcohol that has double bonds between carbon atoms
EXAMPLE: Naming an alcohol that has Double Bond and Substituent Groups
NOTE: Once the main functional group has been assigned, in this case it is the OH group for alcohols, then we do not care about group priorities anymore. That is, they are treated to have equal naming priority. Therefore, we assign the rest of the remaining substituent or functional groups (i.e. Cl and Br in our case) are named based on alphabetical order.
In our case, ‘c’ in chloro comes before ‘m’ in methyl and so we put chloro first in the name of our organic molecule.
Learning Objective #1 - Investigate the IUPAC nomenclature for Aldehydes and Ketones
Use same method (Steps 1 – 5) as we did for naming alcohols.
EXAMPLE: Naming Ketone with Substituent Groups
We will be jumping straight into the deeper end, i.e. naming ketones with substituent groups and skipping the, simpler, nomenclature for ketones without substituent groups.
This is because they all follow the same steps as shown in the previous examples of naming alcohol with and without substituent groups.
There are homework questions on naming both ketones with and without substituent groups in this week’s homework set with solutions so you can practice both and check your answers after your attempt
NOTE: Again, we have already assigned the main functional group being the carbonyl group (C=O) and so the remaining substituent or functional groups (i.e. Br, Cl, OH in our case) are named alphabetically without the need to consider naming priority. That is because they’re all treated as substituent with equal priority.
EXAMPLE: Naming Aldehyde with Substituent Groups
EDIT for typo in above image: For ‘Step 3’, the double bond is at carbon #2 rather than at carbon #3.
The IUPAC name remains correct being But-2-enal.
NOTE: Unlike ketones, aldehydes can only have the carbonyl group (C=O) on carbon number 1 (lowest assigned carbon).
Therefore, it is NOT necessary to write But-2-en-1-al, but, rather we should write But-2-enal (without the “-1-“) as per IUPAC nomenclature rules.
This is because the carbonyl group is always attached to one hydrogen atom and only one alkyl (R) group, rather than two alkyl groups in ketones.
Remember that alkyl (R) groups can have one or more carbon atoms so ketones’ carbonyl group does not necessarily to be on carbon number 1 (unless the R group is a hydrogen atom in the ketone).
Learning Objective #1 - Investigate the IUPAC nomenclature for carboxylic acids
We use the same method (Steps 1-5) to name carboxylic acid as we did for naming alcohols.
EXAMPLE: Naming carboxylic acid with Substituent Groups
NOTE: The carboxyl functional group is always on carbon number #1 in carboxylic acid due to the presence of the hydroxyl group (OH) that is attached to the carbon. Hence, it is NOT necessary to write Ethan-1-oic Acid but rather, we should write Ethanoic acid (without the “-1-“).
For the same reason, since we only have two carbons in ethanoic acid and that hydroxyl group is always attached on one side, it implies that the chloro substituent atom must be always be on carbon #2 in ethanoic acid as we start numbering from the side of carboxyl group.
Therefore, it is NOT necessary to write 2-chloroethanoic acid but, rather, we should write chloroethanoic as per IUPAC nomenclature rules.
Learning Objective #1 - Investigate the IUPAC nomenclature for amine and amides
Same method (Steps 1 – 5) to name amine and amides like we to name alcohols.
EXAMPLE: Naming Amine with Substituent Groups
NOTE: The green arrows illustrates how you can incorporate either one of the two CH3 as your parent carbon chain. It doesn’t not affect your overall name of the organic molecule as the two groups can be rotated around carbon number #2.
NOTE: It is necessary to name “2-methyl“prop-2-en-1-amine (rather than methylprop2-en-1-amine) as the methyl group can be on carbon number #1, i.e. 1-methylprop-2-en-1-amine.
The “-1-amine” is also necessary in the name in our case as there is no fixed atom in amine and but only a R group (R-NH2). Depending on the number of carbon atoms in the R group, it can attach amine to different carbon numbers.
EXAMPLE: Naming Amide with Substituent Group
Notice that in the above example, the amine functional group (R-CO-N-R’-R”) has an alkyl group (i.e. CH3 to be specific).
The above molecule is still an amide as there is a carbonyl group (C=O) attached to a nitrogen atom with two R groups.
NOTE: We have a methyl group attached to the nitrogen atom (rather than carbon) which is not numbered and another methyl attached to carbon number #2. Hence, the “2,N-dimethyl”
Numbers always come before letters which is we have done in previous examples as well as here. For instance, “3-chloro”. So, we put “2,N” rather than “N,2”
Learning Objective #1 - Investigate the IUPAC nomenclature for halogenated organic compounds
These are just organic compounds with halogen atoms attached to them. They are low in substituent group naming priority and so they’re just used as prefixes only.
We have already examined some examples of organic molecules (e.g. alcohols and ketones) with halogens attached to them in the above sections.
The following list are of the halogen atoms that you will encounter in organic molecules during in HSC Chemistry which we have already seen previously.
Chlorine
Bromine
Fluorine
Iodine (uncommon)
Learning Objective #2 - Explore and distinguish the different types of structural isomers, including saturated and unsaturated hydrocarbons.
Saturated hydrocarbons, i.e. alkanes, are those whereby there are only single covalent bonds between carbon atoms in the hydrocarbon molecule.
Comparatively, unsaturated hydrocarbons, i.e. alkenes and alkynes, are those whereby there are double or triple bonds between carbon atoms in the hydrocarbon.
In organic chemistry, structural isomers often called constitutional isomers at University level chemistry.
Structural isomers are used to describe molecules that have the same molecular formula but atoms that make up the molecules are arranged differently along the parent carbon chain structure, thus having different structural formula.
Learning Objective #2 - Explore and distinguish the difference types of structural isomers, including saturated and unsaturated hydrocarbons including: Chain Isomers
Alright, let’s explore the first type of structural isomers – chain isomers!
We can say that two or more molecules are chain isomers if they satisfy the following conditions:
They must have the same molecular formula.
They must differ in their connectivity of carbon atoms to the parent carbon chain.*
*This means that these isomers have different parent carbon chain lengths, hence they have the name being chain isomers.
EXAMPLE: Chain Isomers
We have now examined the definition and criteria required how molecules to be qualified or classified as chain isomers.
We also have seen some examples of chain isomers.
Now, check out the HSC Chemistry Guide below to that takes you through to learn how to draw chain isomers in a step-by-step guide.
Learning Objective #3 - Explore and distinguish the different types of structural isomers, including saturated and unsaturated hydrocarbons including: Position Isomers
All aboard the train! We are going to the next stop to explore another type of structural isomers, position isomers!
In order for two or more molecules to be considered position isomers, they need to meet the following criteria:
Like all structural isomers, position isomers must have the same molecular formula.
Unlike chain isomers, position isomers have the same carbon structure in terms of parent carbon chain length.
One or more substituent and/or functional groups must differ in their connectivity to the carbon atoms along the parent carbon chain.*
*This effectively means that these isomers needs to have one or more of their substituent and/or functional groups being in a different position along the carbon structure. Hence, they have the name being position isomers.
Recall that from the table that we examined in Learning Objective #4, where we showed how double and triple bonds between carbon atoms are functional groups.
So, isomers that differ in their position of double and triple carbon bonds in their carbon structure can be considered position isomers (provided that the molecules satisfy all of other criteria for position isomers outlined above).
EXAMPLE: Position Isomers
View the HSC Chemistry Guide below to see how you can draw position isomers, step-by-step, when given chemical name or chemical formula of an unknown organic molecule.
Learning Objective #4 - Explore and distinguish the different types of structural isomers, including saturated and unsaturated hydrocarbons including: Functional Group Isomers.
We have arrived at the terminal stop on the train in exploring structural isomers!
Note that there are other forms of structural (or constitutional) isomers such as ring-chain isomers, tautomerism and metamerism but they are outside the scope of the HSC Chemistry Syllabus.
Anyways, in this final stop, we will explore functional group isomers!
Again, there are a set of conditions that two or more molecules must satisfy in order for them to be considered functional group isomers. These are:
Like all structural isomers, they must have the same molecular formula.
They may or may not have the same parent carbon chain length, depending on arrangement of atoms.
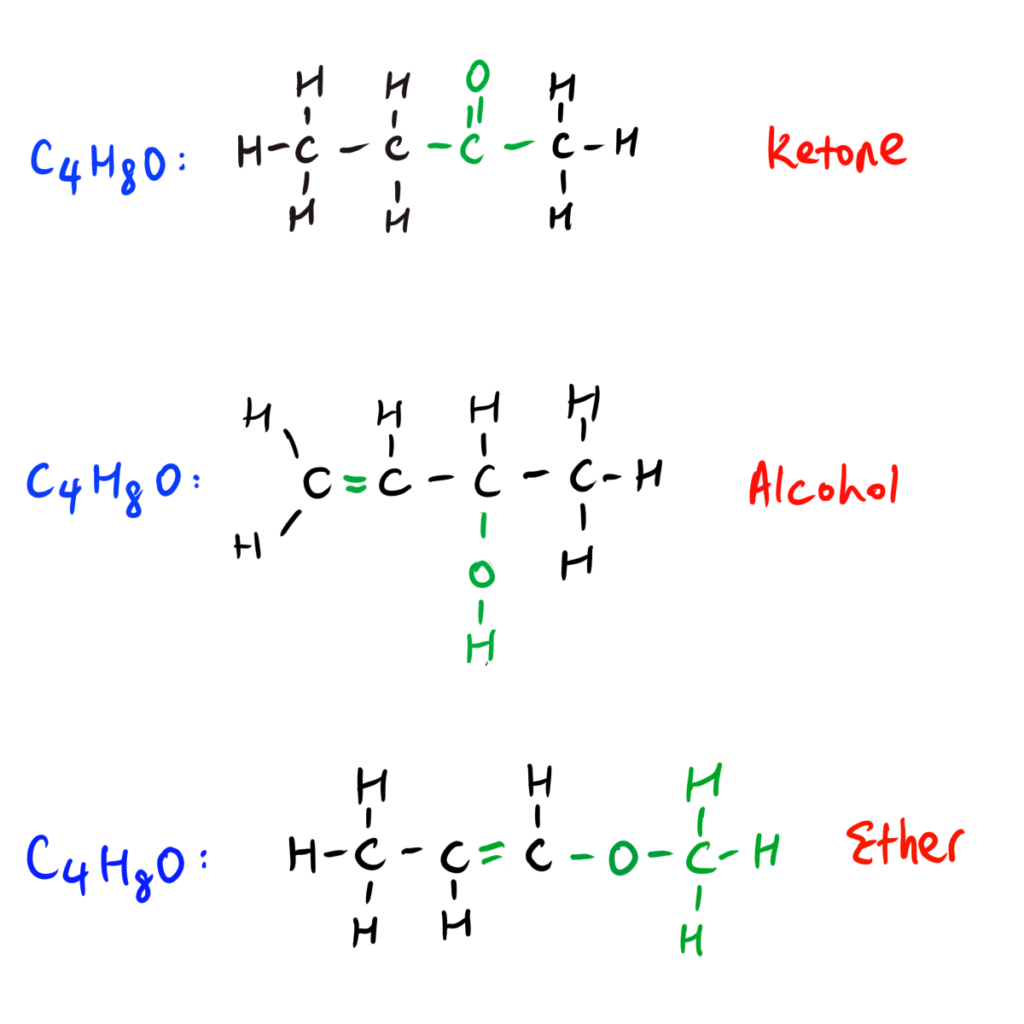
Due to the isomers differing in their arrangement of atoms, (thus, NOT limited to only substituent and functional group like for positional isomers), this results in the isomers having different functional groups.*
*Hence their name, functional group isomers.
So, functional isomers are molecules in different homologous series as they have different functional groups.
EXAMPLE: Functional Group Isomers
Now we know the definition, criteria and have seen some examples of functional group isomers.
Check out the Step-by-Step HSC Chemistry Guide below on how to draw functional group isomers when given chemical formula or name.
Variation in physical and chemical properties between different types of structural isomers
Chemical properties
Structural isomers would have the same chemical property if they have the same functional group(s) in their structural formula.*
*That being said, it is always important to examine the structure formula of molecules to determine whether any chemical reactions that you have learnt in HSC Chemistry which may take place.
This effectively means that only functional group isomers would have different chemical properties.
As you have learnt in Preliminary HSC Chemistry, a different functional group provides a distinctive chemical property to the molecule to undergo certain chemical reactions.
Physical properties
The following guidelines will help you compare the melting and boiling points between different isomer molecules.
As you will explore with polymers which is part of the last Inquiry Question in Module 7, you learn that molecules (e.g. isomers) that exhibit a less branched (or linear) structural formula have a higher melting and boiling point.
Therefore, you would expect the more chain branching that a molecule exhibits, the lower its melting and boiling point will be.
This is because chain branching prevents the isomers from packing their carbon chains closely together and, thus, the intermolecular forces (e.g. dispersion forces) between them would be weaker. As we have explored in Preliminary HSC Chemistry, intermolecular forces play an important role in governing the melting and boiling point of molecules.
As for functional group isomers, the isomers have different functional groups allowed differing extent of chain branching (thus affecting dispersion forces) and maybe different degree of dipole-dipole and hydrogen bonding. Therefore, their melting and boiling point (physical property) may vary.
Example: Alcohols (- OH) and carboxylic acids (- COOH) have different melting and boiling points due to different functional groups.