HSC Chemistry Syllabus Notes
Module 7 / Inquiry Question 2
Overview of Week 9 Inquiry Question – How can hydrocarbons be classified based on their structure and reactivity?
Learning Objective #1 – Construct models, identify the functional group, and write structural and molecular formulae for homologous series of organic chemical compounds, up to C8:
– alkanes
– alkenes
– alkynes
Learning Objective #2 – Conduct an investigation to compare the properties of organic chemical compounds within a homologous series, and explain these differences in terms of bonding.
Learning Objective #3 – Analyse the shape of molecules formed between carbon atoms when a single, double or triple bond is formed between them.
Learning Objective #4 – Explain the properties within and between the homologous series of alkanes with reference to the intermolecular and intramolecular bonding present.
Learning Objective #5 – Describe the procedures required to safely handle and dispose of organic substances.
Learning Objective #6 – Examine the environmental, economic and sociocultural implications of obtaining and using hydrocarbons from the Earth
NEW HSC Chemistry Syllabus Video – Hydrocarbons
Week 8 Homework Questions (Essential for Band 5!)
Week 8 Curveball Questions (Moving from Band 5 to Band 6!)
Week 8 Extension Questions
Solutions to Week 8 Questions
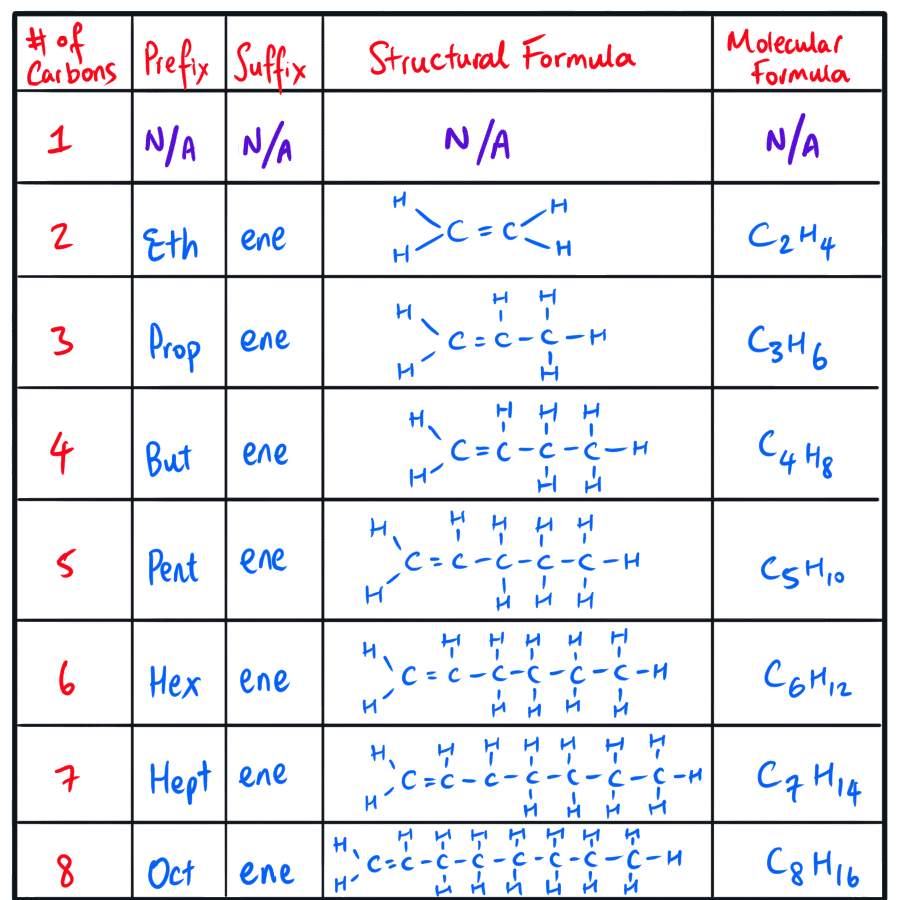
Learning Objective #1 - Construct models, identify the functional group, and write structural & molecular formulae for homologous series of organic chemical compounds, up to C8 for the following categories of hydrocarbons:
What is a homologous series?
A homologous series is just a category of molecules with the same functional (sometimes substituent e.g. in alkanes) group.
The term ‘homologs’ or ‘homologues‘ refers to compounds that are in the same homologous series e.g. methane, ethane, propane are homologs/homologues as they are all part of the same homologous series called Alkanes.
Each previous and subsequent homolog differs in the number of only one CH2 unit.
Each homolog has the same chemical properties as they have the same functional group.
Due to their different molecular mass, they have different physical properties (e.g. molecular mass, melting and boiling point, density, viscosity, etc). The reason for this we will explore in Learning Objective #2 and #4 later in this week’s notes.
We will now proceed to explore various homologous series of hydrocarbons. If you recall from last week’s we said that hydrocarbons are compounds that solely have carbon and hydrogen atoms and no other atoms.
Neat! Let’s go explore
Hydrocarbon Homologous Series Type #1: Alkanes
Just like a merry-go-round, here we are back to the alkanes homologous series again.
Here, we will explore how to write out a structural and molecular formula for alkanes when we given the name of an alkane such as ethane.
There is a general formula to assist us in doing this. This formula is: CnH(2n+2), where n is the number of carbon atoms that the alkane has.
NOTE: This formula works well for alkanes that does not have any substituent group as the formula would fail when there are substituents in place. Refer to last week’s notes if you are unsure of the definition of substituents and functional groups.
Hydrocarbon Homologous Series Type #2 - Alkenes
Just like we have a formula for naming alkanes without substituent groups, we also have one for naming alkenes without substituent groups! How great, yay
The formula that is of our concern here is: CnH(2n), where n is the number of carbon atoms that the alkene has.
Again, this is only for alkenes that have do not have substituent groups.
NOTE: All of the above alkenes that are drawn above have the C=C bond located at the first carbon.
For example: the precise alkene names would be: Prop-1-ene, But-1-ene, Pent-1-ene, etc.
NOTE: The molecule, methene, does not exist.
NOTE: There is no need to number the position of the C=C in ethene because there are only two carbon atoms in the parent carbon chain. That is, there is no need for eth-1-ene but just ethene.
NOTE: IUPAC actually prefers the name ‘ethylene’ rather than ‘ethene’.
Hydrocarbon Homologous Series Type #3 - Alkynes
Lastly, we will explore the formula for naming alkynes without substituent groups.
The formula to name these alkynes is: CnH(2n-2).
NOTE: All of the above alkynes have the triple carbon bond at the first carbon.
For example: the precise alkyne names would be: Prop-1-yne, But-1-yne, Pent-1-yne, etc.
NOTE: Again, as there are only two carbon atoms in ethyne parent carbon chain, this means that the triple bond can only be between those two carbons so there is no need to use -1- to specify the location of the triple bond.
Learning Objective #2 - Conduct an investigation to compare the properties of organic chemical compounds within a homologous series, and explain these differences in terms of bonding.
We have already discussed a whole list of different homologous series in last week’s notes. For recap purposes, let’s explore the list of various homologous series that we examined last week.
The following list of homologous series are ordered based on decreasing naming priority of functional groups, similar to how we assorted them in last week’s notes.
NOTE: In the above table, we left out alkanes by accident. Similar to alkyl halides or halo-alkanes, if the alkane is the main ‘functional group’ (technically substituent group), then it will have the suffix ‘ane’.
If the alkane is substituent, then it has the prefix of the appropriate alkyl group which we explored and examine in last week’s notes.
NOTE: We have already explored how each homologous series have a suffix which allows us to determine which homologous series that it belongs to.
General formula for: Alcohol Homologous Series is: Cn(2n+1)OH
General formula for Aldehyde Homologous Series is: CnH(2n+1)CHO
General formula for Ketone Homologous Series is: CnH(2n+2)CO
General formula for Carboxylic acids Homologous Series is: CnH(2n+1)COOH
General formula for organic amide homologous series: R-CO-NH2
General formula for organic amine homologous series: R-NH2
Homologues in the Alkane Homologous Series (without substituent groups)
As we have learnt in the HSC Preliminary Chemistry Course, the type (and thus strength) of intermolecular forces plays a major role in determining the melting and boiling point of molecules.
This is not limited to just alkanes but for all the compounds in each class of homologous series which we will explore soon.
Here, we will talk about alkanes without substituent groups.
We have already explored the structure of unbranched alkanes without substituent groups in last week’s notes as well as in this week’s learning objective #1. They only have of C-C and C-H bonds which are non-polar bonds due to the electronegativity difference between C-C is basically zero and the electronegativity difference between carbon and hydrogen in the C-H bond not being high enough to be considered a polar covalent bond.
Recall that we have already learnt the electronegativity difference ranges that allows us to classify non-polar, polar and ionic bonds in Preliminary HSC Chemistry.
For such reason, as both C-C and C-H bonds are non-polar in addition to the fact that unbranched alkanes are linear, it allows uniform distribute of any dipole moments, it is justifies why unbranched alkane molecules without substituent groups are non-polar.
Since they are non-polar, it is only dispersion forces being the intermolecular force that is determining the melting and boiling points of unbranched alkanes without substituents.
NOTE: We have already talked about dispersion forces in Preliminary HSC Chemistry, so we expect you to know how they are created. But, just for a quick recap on what they are, dispersion forces are intermolecular forces created due to the uneven distribution of electrons, thus even electron density, around the nucleus of an atom. This therefore results in a small dipole that is formed where one side of the atom is slightly positive and the other side being slightly negative. This dipole induces a similar dipole in a neighbour atom, resulting in a dispersion force being created between the two atoms.
This means alkanes with larger molecular mass will have more atoms (carbon and hydrogen) and hence will have a greater number of electrons. Thus, the degree (and thus strength) of dispersion force formed between larger alkane molecules would therefore be greater than between smaller alkane molecules.
So, a higher amount of energy is required to break the intermolecular forces (dispersion forces only between alkane molecules) in alkanes with higher molecular mass.
Therefore, as the number of carbon and hydrogen atoms increase (i.e. alkane molecule increases in molecular mass – such as going from methane to octane), the melting and boiling point will increase.
As you are aware from Preliminary HSC Chemistry, hydrocarbons from C1 to (and including) C4 are gases at room temperature. Hydrocarbons between C4 – C8 are liquids at room temperature.
Branched vs Unbranched Alkanes
For two alkanes, one being branched and the other unbranched, both with the same molecular formula and thus molecular mass. The unbranched alkane will exhibit higher melting & boiling point. The reason for this is because unbranched alkanes are more linear whereas branched alkenes are less linear (or more spherical or more bulky) in shape.
For such reasons, the carbon chains between unbranched alkane molecules are able to pack more closely together than the carbon chains between branched alkanes. Therefore, a higher surface contact area means that the extent of dispersion force is greater between unbranched alkanes as more atoms’ electrons are able to interact with each other to induce dipoles. This results in unbranched alkanes having a higher melting & boiling point.
Another differing physical property other than melting & boiling point between alkanes of different molecular weight is their density. Their density increase as the molecular weight increases, however, it doesn’t exceeds the density of water. Obviously, another density trend is that, since unbranched alkanes have their carbon chains packed more closely with each other, they have higher density than branched alkanes.
With that, we conclude the physical property differences between homologues in the alkane homologous series!
General physical properties of all alkanes share are:
Alkanes are not soluble in polar substances but soluble in non-polar substances (the reason for this is already covered in HSC Preliminary Course, so we won’t re-explain here).
Alkanes are colourless and odourless.
NOTE: As we have mentioned previously, the chemical properties shared between homologues in a homologous series such as alkane are the same since there is no different atoms or functional groups.
Homologues in the Alkene Homologous Series (without substituent groups)
The differences between alkene molecules of increasing molecular mass is similar to the case of alkanes that we described above already. So, we will only do a very brief summary.
Very brief summary (as the case of alkenes is similar to alkanes which we explained in detailed above): As the molecular mass of alkenes increases, the strength of dispersion forces increases and thus results in a trend of increasing melting & boiling point with increased molecular mass.
It is important to note that alkenes have a lower melting and boiling point than their corresponding alkane with the same number of carbon atoms. This is because alkenes have a lower molecular mass, lowering the strength of dispersion force.
For the same reason, alkenes have a lower molecular than corresponding alkanes with the same number of carbon atoms.
NOTE: Both alkanes and alkenes have less density than water as well as being insoluble.
Similar to alkanes, the alkenes from C1 up to and including C4 exist in the gas state at room temperature whereas alkenes that is C5 – C8 are liquids.
In general, apart from melting & boiling point, alkenes share similar physical properties to alkanes are they are comprised of the same types of atoms, carbon and hydrogen only.
The chemical properties shared between alkene homologues are same as there is no difference in functional groups.
Homologues in the Alkynes Homologous Series (without substituent groups)
The physical properties are the same as alkenes and alkanes. However, alkynes have lowest melting & boiling point out of the three due to lower molecular mass than corresponding alkane and alkene hydrocarbons with the same amount of carbons.
In reality, low weight alkynes have highest melting and boiling point but for HSC Chemistry purposes, the trend that you will be looking at should be Alkane > Alkene > Alkynes in terms of M.P and B.P and explain them in terms of strength of dispersion force.
Alkyne homologues share similar with each other chemical properties as they all have a triple bond between carbon atoms.
Difference in chemical properties between Alkanes, Alkenes & Alkynes without substituent groups
As alkenes and alkynes have double and triple bonds between carbon atoms, they are more reactive than alkanes. This is because there is higher electron density in a double and triple bond region compared to single bonds meaning that breaking a C-C bond from double bond and triple bond between carbon atoms require less energy to break compared to breaking the one and only single carbon-carbon bond between carbon atoms.
This therefore allows alkenes and alkynes to undergo addition reaction whereby a carbon-carbon bond is broken so that a substituent group or atom can be added to each carbon.
Alkanes cannot undergo addition reactions but instead undergoes substitution reactions as they only have a single bonds, i.e. C-C and C-H.
A common test for alkanes and alkenes is bromination (a type of halogenation reaction whereby Br2 is added to the organic compound).
What happens is that alkanes will not react with the bromine molecule unless under the presence of high temperature or U.V light as we will discuss next week under halogenation, subsititution reactions.
Comparatively, addition reaction occurring between alkenes and alkynes with bromine does not need high energy due to lower bond energy of breaking of one of the several C-C in a double bond or triple bond between carbons atoms. Therefore, the bromine molecule will be incorporated into the alkenes and alkyne molecules via addition reaction at room temperature.
Since bromine has a red-brown colour and the hydrocarbons (e.g. alkane, alkene, alkynes) are colourless, the effect is that when bromine undergoes addition reaction or substitution reaction, there will no longer be bromine molecules in solution and thus the solution will become colourless after reaction.
For alkynes, it basically is the same as alkenes in terms of chemical properties but they are more reactive than alkenes (and alkanes) as two C-C bonds can be broken with lower energy in its triple carbon bonds between carbon atoms. Therefore, alkynes can undergo addition reaction twice to be compared into an alkene which can only undergo only one addition reaction.
In general, this means that alkynes are less stable than alkenes which are less stable than alkanes.
In order of acidity, alkynes are the most acidic: i.e. alkynes > alkenes > alkanes.
That is alkynes are the strongest acid as they are readily donate their hydrogen atom in the form of H+.
The reason towards this trend of acidity is because the resulting lone electron pair on the carbon atom, after a hydrogen atom is donated is situated in the sp orbital compared to the sp2 in alkenes and sp3 in alkanes.
Recall from Preliminary HSC Chemsitry:
Sp orbital have 50% s character and 50% p character.
Sp2 has 1/3 s character and 2/3 p character.
Finally Sp3 orbitals have 25% s character and 75% p character.
As electrons that are closer to the nucleus (more s character) are more stable than electrons found further away from the nucleus (less s character) due to higher effective nuclear charge, the reaction of alkynes acting as a Bronsted-Lowry Acid by donating a H atom occurs more readily compared to alkenes and alkanes as the conjugate base formed is more stable. This is because the conjugate base’s lone pair of electron have greater s character in alkynes than alkenes and alkanes which experiences a higher effective nuclear charge and thus is more stable as the electrons are closer to the nucleus which is positively charged.
Finally, alkanes have higher chance of undergoing complete combustion than alkenes which have higher chance than alkynes. This is because the ratio between carbon to hydrogen is higher in alkynes than alkenes which is higher than alkanes so more oxygen is required to fully oxidise the carbon atoms.
Most of the oxygen are used to form water and any remaining is used to form either carbon dioxide (two oxygen per carbon) or carbon monoxide (one oxygen per carbon), where carbon monoxide is favoured if there is limited availability of oxygen.
Homologues in the Alkyl halides (or Haloalkane) Series
The electronegativity difference between the halogen and the carbon atoms makes the C-X bond polar, where X represents a halogen atom. Due to this, there are dipole-dipole forces between haloalkane molecules as well as dispersion forces compared to only dispersion forces present between molecules in the three hydrocarbon homologous series (alkane, alkenes and alkynes).
For such reasons, haloalkanes have stronger intermolecular forces than corresponding alkanes/alkenes or alkynes with the same number of carbon atoms. Again, similar to other homologous series, as haloalkanes increases in alkyl group chain length, the molecular mass will increase. This results in higher extent of dispersion forces due to greater number of electrons, resulting in stronger intermolecular forces.
Another similar trend is that, as the length of the non-polar alkyl group increases, the solubility of haloalkanes in polar substances decreases due to increasing non-polar nature of the overall alkyl halide.
Do note alkyl halides cannot perform hydrogen bonding with water molecules so they are not readily soluble in water. So, alkyl halides are less soluble than corresponding ketones, aldehydes, alcohols, amides and amines with the same number of carbon atoms (similar molecular mass) that can perform hydrogen bonding with water molecules.
Alkyl Halide Density:
For alkyl halides that are homologues, as the number of carbon increases, the molecular mass increases. That’s a pretty simple physical property trend that you can apply to all other homologous series.
A thing to note about alkyl halides is that as its molecular mass increases (i.e. moving from C1 to C8), the density of the homologues in the series decreases (measured at a fixed temperature) as the ratio between mass to volume for density decreases.
However, if the alkyl chain is the same length between two haloalkanes, the one with the heavier or higher molecular mass halogen will result in the haloalkane being more denser.
Most alkyl halides exists as a liquid at room temperature except for few of those with low molecular mass such as fluoromethane and chloromethane.
Homologues in the Ketone Homologous Series
Compared to aldehydes, ketone molecules have a pleasant and sweet odour.
Due to the electronegativity difference between the oxygen and carbon atom in the C=O group, the carbonyl group is polar. This essentially gives ketone molecules their polar properties.
The main intermolecular force that determines the melting & boiling point of ketone molecules are dipole-dipole forces. Obviously, dispersion forces are present amongst the molecules too.
Ketones have a higher boiling point than aldehydes because ketones have two alkyl (R) groups attached a central carbon atom where each alkyl group has a greater electron density than a hydrogen atom. Comparatively, aldehyde only has one alkyl (R) group and one hydrogen atom attached to a central carbon atom.
Recall that since the C=O bond is polar due to the oxygen atom being more electronegative than carbon atom. This means that oxygen is partially negative and the carbon atom is partially positive.
This means that two alkyl groups (each has higher electron density than a hydrogen atom) will attracted and be located closer to the central carbon atom. This effectively makes the central carbon atom in ketones more negatively charged, resulting ketones having a more polarised C=O bond compared to that C=O bond in aldehydes.
A more polarised C=O bond in ketones means that the dipole-dipole forces between ketone molecules are stronger than those between aldehyde molecules. This means that more energy is required to break the dipole-dipole intermolecular forces between ketone molecules than between aldehydes.
For such reason, ketones have a higher boiling point than aldehydes.
Although the ketones with one to three carbons (C1 to C3) are very soluble in water, as the molecular mass increases (i.e. moving into C4 and beyond), the length of the non-polar hydrocarbon chain increases. This results in ketone molecules experiencing a gradual decrease in water solubility as molecular mass increases, resulting in the non-polar alkyl chain/group increasing in length.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the ketone homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Homologues in the Aldehyde Homologous Series
Compared to ketones, aldehydes have an unpleasant and strong/sharp odour.
Aldehydes are polar for the same reason (for the most part) as Ketone as explained in the ketone section.
The main intermolecular force between aldehyde molecules are dipole-dipole forces, followed by dispersion forces.
If you pursue higher chemistry at university, you will learn that aldehydes can in fact hydrogen bond with themselves at certain configurations but that’s outside the scope of HSC Chemistry. To limit to the scope of HSC Chemistry scope, aldehydes and ketones cannot form hydrogen bonds with each other. They can form hydrogen bonds with other molecules such as water though!
You can try draw out the structures to see how ketones and aldehyde can hydrogen bond with water as we have learnt about how hydrogen bonding can occur in different situations in Preliminary HSC Chemistry.
Aldehydes have a lower melting and boiling point than ketones where the reason why has been explained in the previous section on ketones.
Although the aldehydes with one to three carbons are very soluble in water, as the molecular mass increases (i.e. moving into C4 and beyond), the length of the non-polar hydrocarbon chain increases. This results in aldehydes experiencing a gradual decrease in water solubility as molecular mass increases, resulting in the non-polar alkyl chain/group increasing in length.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the aldehyde homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Homologues in the Alcohol Homologous Series
Like homologues in any homologous series, the homologues in the the alcohol homologous series differ by one carbon and one CH2 unit. This difference in molecular size that gives homologues in the alcohol homologous series their different physical properties. Since all homologues in the alcohol homologous series have one OH functional group, their chemical properties are the same.
The main and strongest intermolecular force between homologues in the alcohol homologous series is hydrogen bonding. Of course, as you have learnt in Preliminary HSC Chemistry, molecules of a compound that are capable of hydrogen bonding amongst each other means that they will also have dipole-dipole as well as dispersion forces.
Here, the learning objective is concerned about the properties between homologues in the alcohol homologous series. We know that the intermolecular forces between molecules of a compound plays a major role in determining the physical compound’s properties from Preliminary HSC Chemistry.
Hydrogen bonding is stronger than dispersion forces as the electronegative difference between the atoms involved in creating the intermolecular force is greater than in generic dipole-dipole forces.
Quick recap from Preliminary HSC Chemistry on hydrogen bonding: For a hydrogen bond to be formed, a highly electronegative atom must be added to a hydrogen, creating a slightly negative charge on the more electronegative atom and slightly positive charge on the hydrogen. The slightly positive charged hydrogen atom can perform hydrogen bonding with another electronegative atom (slightly charged) from another molecule as long as the electronegativity difference is strong enough.
For such reason, alcohols have higher melting & boiling point than the hydrocarbon homologous series (i.e. alkanes, alkenes and alkynes) that do not have hydrogen bonds and only dispersion forces.
Another factor that contributes towards alcohol’s higher boiling point is due to the existence of a hydroxyl group (OH) that replaced a hydrogen in the hydrocarbon homologous series (e.g. in alkanes).
As you have learnt in Preliminary HSC Chemistry, the strength of dispersion force is proportional to electron number, an hydroxyl group (OH) has an extra oxygen atom (thus more electrons) compared to a hydrogen atom. Therefore, the strength of dispersion forces is also greater in alcohols than in homologues in hydrocarbon homologous series like alkanes.
NOTE: Since homologues in the Aldehyde and Ketone categories only has dipole-dipole forces and not hydrogen bonding, corresponding alcohol molecules with the same number of carbon atoms (i.e similar molecular mass) would therefore have higher melting & boiling points.
Although the alcohol molecules with one to three carbons (C1 to C3) are soluble in water, as the molecular mass increases (i.e. moving into C4 and beyond), the length of the non-polar hydrocarbon chain increases. This results in alcohols experiencing a gradual decrease in water solubility as molecular mass increases due to the increasing non-polar alkyl chain/group.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the alcohol homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Lastly, as the molecular mass increases (due to increase carbon and hydrogen atoms) between alcohol homologues, the viscosity of the homologues increases. This is because the the larger molecular size of the alcohol molecules increases the extent of dispersion forces as well as the large size provide resistance in hindering alcohol molecules from escaping the liquid surface. This trend of viscosity can be applied to other applicable organic molecules that are in the liquid state in different homologous series.
Amide Homologous Series
Apart from the amide that has only one carbon (C1) in the homologous series existing as a liquid at room temperature, i.e. methanamide, all the rest of the other homologues in the amide homologous series are solids at room temperature.
The main intermolecular force that determines the melting & boiling point of amides is hydrogen bonding.
Compared to alcohols and carboxylic acids with the same number carbon of atoms, the corresponding amide has a higher melting & boiling point. This is because amides have the C=O bond as well as the two N-H polar bonds that can hydrogen bonds.
As you will learn soon, all of the homologous series that we will talk and have talked about in this learning objective will have a lower density than water except for amides! This isn’t surprising considering amides are solids at room temperature except the amide with one carbon atom (C1), i.e. methanamide.
An identical trend to homologues in other series, as the number of carbon in parent chain increases (from C1 to C8), molecular mass will increase.
In terms of solubility, this is similar to other cases we have talked about. As the molecular mass increases, the non-polar alkyl group will increase resulting in a gradual decline in solubility in polar substances.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the amide homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Amine Homologous Series
Amines in the gas phase have an ammonia odour. Comparatively, liquid amines have a “rotting fish” odour.
Amines with one and two carbons (C1 and C2) are gas at room temperature – i.e. methanamine and ethanamine. The other amines from C2 to C8 are liquids at room temperature.
The primary intermolecular force that governs the melting and boiling point of amines is hydrogen bonding.
This is because the N-H bond is a polar covalent bond as the nitrogen atom is much more electronegative than the hydrogen atom.
For such reasons, hydrogen bonding exists between amine molecules. If you draw out an amine molecule, you can see that one primary amine molecule has the capacity to perform hydrogen bonding with two water molecules.
In terms of melting and boiling point, alcohols and carboxylic acids have a higher melting & boiling point than amines. This is because the O-H bond is more polar than the N-H bond as oxygen is more electronegative than nitrogen.
Similar to alcohols, as the molecular mass of the amine molecules increases, the length of the non-polar alkyl chain increases. Thus, the solubility of amines molecules in polar substances (or solvents) such as water decreases with increasing molecular size.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the amine homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Amine vs Amide chemical test:
• Since amine are basic and amides are not, you can react amide with a strong base such as NaOH to form ammonia gas and a salt (such as R-C=O-Na). So, you can see bubbles forming due to evolution of ammonia gas. There will be no bubbles formed when you attempt to react amine and NaOH as there is no reaction between base reacting with another base, provided that their concentrations are not extremely high.
Carboxylic Acid Homologous Series
The main intermolecular force that determines the melting & boiling point between homologues in the carboxylic acid homologous series is hydrogen bonding.
As seen from the carboxylic acid functional group (COOH) structure we have explored in last week’s notes, we can see that there is a carbonyl group (C=O) and a hydroxyl group (O-H). So, each carboxylic acid group has the capacity to form two hydrogen bonds with another carboxylic acid molecule.
Comparatively, when we compare carboxylic acids to alcohols, ketone and aldehydes, they can only form one hydrogen bond. Therefore, carboxylic acids have higher melting & boiling point compared to their corresponding counterparts in other homologous series with the same number of carbon atoms except for amide (which has the highest).
Similar to alcohol, although the carboxylic acids with one to three carbons are soluble in water, as the molecular mass increases (i.e. moving into C4 and beyond), the length of the non-polar hydrocarbon chain increases. This results in carboxylic acids experiencing a gradual decrease in water solubility as molecular mass increases due to the increasing non-polar alkyl chain/group.
That being said, when comparing carboxylic acid to their corresponding alcohol with the same number of carbon, the carboxylic acid is more soluble in water due to the capacity to form two hydrogen bonds compared to only one for alcohol.
Like all of the previous cases, as the number of carbon and hydrogen atoms increases (i.e. molecular mass) for homologues in the carboxylic acid homologous series, the strength of dispersion force increases. Thus, the melting & boiling point increases.
Summary of common trends amongst homologues in various homologous series
We talked about the trend that melting & boiling point increases with the size of homologues (C1 to C8) within and between homologous series.
General trend of decreasing melting & boiling point:
Amide > Carboxylic Acid > Alcohol > Ketone > Aldehyde > Amine > Haloalkane > Alkane > Alkene > Alkyne.
(NOTE: the trend should actually be Alkyne > Alkane > Alkene BUT for HSC Chemistry purposes, you should use the trend, Alkane > Alkene > Alkyne and explain it in terms of strength of dispersion force).
We also talked about solubility decreasing with increasing molecular mass.
We also talked about increasing molecular mass with increasing number of carbon in parent chain assuming no branching.
We also differentiated amides against amines in terms of odour. Likewise, aldehydes with ketone with odour.
We differentiated some homologues in homologous series by their states at room temperature.
Learning Objective #3 - Analyse the shape of molecules formed between carbon atoms when a single, double or triple bond is formed between them
Single carbon-carbon bond
An example of a molecule that comprises the single carbon-carbon (C-C) bond can be found in ethane, C2H6.
All alkanes have single carbon-carbon bonds except for methane.
The shape of carbon atoms in ethane have a tetrahedral shape according to VESPR theory since each carbon in ethane has four chemical bonds. Each carbon makes three C-H bonds and one C-C each with a 109.5 degree bond angle.
NOTE: Drawing lewis structures is important to understanding and predicting molecular shapes which we have touched on when exploring the VESPR theory in the HSC Preliminary Course.
NOTE: Since we are dealing with molecules with more than one central atom (two or more atoms bonded to it) such as ethane, most of the time there may not be no single shape that can be used to describe the shape of the full molecule if the central atoms have differing amount of atoms attached to them. In our case, we only have two central carbon atoms in ethane and both carbon atoms have the same amount (4) of atoms attached to it so they have the same shape. So, ethane can be described using one shape, it has a tetrahedral shape around its central carbon atoms. It is important to refer to the shape of the central atoms as we have multiple central atoms for ethane (two carbon central atoms).
NOTE: If you decide to take a first year chemistry course at university, you will realise that there is a difference between geometry and shape when describing molecules where geometry takes into account of lone electron pairs which we won’t and don’t have here.
Double carbon-carbon bond
Ethylene is an example of a molecule that consists of a double carbon-carbon (C=C) bond, C2H4.
All alkenes have double carbon-carbon bonds.
The shape of the central carbon atoms in ethylene have a trigonal planar shape, according to VESPR theory, since each carbon atom makes two C-H bonds and one C-C, each with are approximately 120 degree bond angle.
Since both central carbon atoms have the same shape, ethylene has a trigonal planar shape around its central carbon atoms.
Triple carbon-carbon bond
The molecule Ethyne is an example of a molecule that consists of a triple carbon-carbon bond, C2H2.
All alkynes have triple carbon-carbon bonds.
The shape of the central carbons ethyne is linear according to the VESPR theory since each carbon atom has one C-H and one C-C bond which each has 180 degrees bond angle.
Since both central carbon atoms have the shape, ethyne has a linear shape around its central carbon atoms.
Learning Objective #4 - Explain the properties within and between homologous series of alkanes with reference to the intermolecular and intramolecular bonding present
Learning Objective #4 has been covered in Learning Objective #2, please refer to it.
Learning Objective #5 - Describe the procedures required to safely handle and dispose of organic substances.
Below are some common laboratory practices when dealing with organic substances. We will go through ten laboratory practices which you probably only need to write down three for HSC purposes.
Handling – i.e. storing, using and treating spills of organic substances
I. Store “like material with like” – so that flammable materials should be stored together and away (in a different cabinet) from materials that are corrosive, toxic, etc to prevent hazardous reactions.
II. Oxidising agents must be stored separately from organic substances as they can ignite organic solvents or acids.
III. Organic nitrates and peroxidies are shock-sensitive and should be handled with care as shock can result them in generating large volume of gases in a short period of time, resulting in a high increasing pressure. This would result in an explosion.
IV. Some organic acids such as acetic and propanoic acid are corrosive which can cause burns upon contact with skin where severity depends on concentration of the acid.
To minimise this risk, a laboratory coat is worn to as an additional protective layer against corrosive substances for the skin (arms, body & thighs).
Safety gloves are worn to protect skin from being in physical contact with corrosive substances in case of spill.
Safety googles are worn to prevent corrosive organic acids from being in contact with eyes in case of splashes.
Enclosed leather shoes are worn to protect feet in case of accidental spills rather than open shoes.
V. Ensure that a well-ventilated area such as a fume hood is used when handling flammable, volatile organic substances (e.g. alcohols). This is because the inhalation of alcohol can cause dizziness and could result in brain damage depending on duration of exposure.
Ensure that the fume hood is closed when not in used to prevent escape of volatile, flammable vapour from the organic substance.
Ensure that there is no open naked flame near the flammable organic substances like alcohols.
VI. When you wish to diluting concentrated acids, you should always slowly add acid to water (of larger volume) and not the other way around. That is, never attempt to dilute acid by adding water to the acid. This is because adding acid to water (of larger volume) allows the water molecules to absorb the heat generated in the dissolution reaction (water also has a higher specific heat capacity than acids). So, it minimises the concentration of heat in a particular area which would otherwise result in splashing of water that contains the acids. The acid may end up in your eye, skin, etc which is undesirable and dangerous.
If you do it the other way around (adding water to acid), the first water molecule that makes contact with the acid will react form hydronium ions which generates a big release of heat. This would cause the water to vapourise or splash which contains the dissolved acid coming at you which you don’t want. Therefore, never add water to acid when diluting but the other way around adding acid to water. “A comes before W”
VII. To clean up an organic substance spill (e.g. organic vapour from, a half mask air-purifying respirator is used. A full-face air-purifying respirator is required if the spilled organic substance is a known eye irritant.
VIII. Wash your hands before leaving the laboratory, even if gloves are worn as a safety precaution especially after cleaning up a spill. This is because organic solvents can strip off your skin’s natural oil layer resulting in skin irritation whereby your skin would be more susceptible to absorbing toxic chemicals.
Disposing organic substances
IX. Ensure that all flasks containing organic substances are labelled such that they can be disposed into the right waste container. All waste containers must also be labelled appropriately such that the correct disposable method can be used.
Liquid and solid organic waste are required to be segregated into different waste containers. Also, inorganic and organic liquid wastes are required to be disposed in separate waste containers.
X. Do not dispose organic substances through the sink as they will contaminate in the waters in which aquatic organisms reside. Organic substances are toxic when ingested by aquatic organism and potentially humans or any terrestrial mammals that consume the water.
Ideally, separate organic and aqueous wastes (mainly water) when disposing organic liquid wastes to reduce the cost of disposal.
Learning Objective #6 - Examine the environmental, economic and sociocultural implications of obtaining and using hydrocarbons from the Earth
Source of hydrocarbons
Hydrocarbons, as we have defined already, as compounds that consists of only carbon and hydrogen atoms.
There are many two classes of hydrocarbons. These are acyclic and cyclic hydrocarbons.
Acyclic hydrocarbons are primarily derived from petroleum and natural gas. These hydrocarbons have a linear structure. Alkanes, Alkene, Alkynes that we have explored in last week’s and this week’s notes are examples of acyclic hydrocarbons.
Comparatively, cyclic hydrocarbons are generally sourced from coal. These hydrocarbon have a cyclic or circular structure as they have a hexagonal-shaped phenyl ring, C6H5, which has three alternating C=C bonds.
Let’s do a minor junior science recap before we get into the crux of the different hydrocarbons derived from petroleum, natural gas and coal.
Petroleum and Natural Gas can be found at different levels deep in the Earth’s crust. Petroleum and Natural Gas formed as dead aquatic organism are covered in sand, clay and mud at the bottom of the sea. Due to the environment consisting of the lack of oxygen, high heat and high pressure, the dead matter from the organisms are converted into petroleum and natural gas.
As petroleum is lighter than water, it is situated above water. Comparatively, the natural gas is formed above the petroleum layer but then becomes trapped between the impervious rocks that is above it. Therefore, natural gas can be sourced from the impervious rocks that are located deep in the Earth’s crust.
Coal is formed by the decomposition of dead plants and trees on land covered in soil and mud. Over time, the dead matter is trapped and subjected to an environment that has no oxygen (anaerobic), high heat and high pressure. Thus, coal is sourced deep within the Earth’s curst.
Now with that introduction out of the way, let’s address the hydrocarbons that are derived from in petroleum, natural gas and coal!
Petroleum mainly consists of hydrocarbons from C1 to C30 that are used in everyday life or industrial processes. For example:
Petrol used in motor vehicles have hydrocarbons mainly consisting of 8 carbons (octane)
Paraffin wax used in candles & machine lubricants consists of hydrocarbons with 20 carbons or higher.
Natural gas mainly consists light weight hydrocarbon such as methane (~80%) which has the molecular formula, CH4. Other light weight hydrocarbons include ethane, propane and butane.
Yes, these unbranched hydrocarbons from C1 to and including C4 (e.g. butane) are all gases at room temperature as we have pointed out in Preliminary HSC Chemistry.
Coal mainly consists of carbon rich compounds which can be burnt in an anaerobic environment to produce methane.
Environmental Implications
As we have mentioned previously, petroleum has a wide range of hydrocarbons with differing molecular weights from C1 to C30 (or even higher). Depending on the hydrocarbon’s physical & chemical properties, amount of hydrocarbon and time of contact, the hydrocarbon’s effect of the environment can vary upon contact.
It is important to note that only a small portion of the wide range of hydrocarbons in petroleum actually do yield harmful effects (e.g. toxic) to the environment.
Let’s first explore how obtaining hydrocarbons can result in environmental implications!
Obtaining petroleum and natural gas from the Earth results drilling through rocks deep in the Earth’s crust whereby hydrocarbons from the drill machine’s lubricants can be dispersed into surrounding water pollutes the surrounding seawater or ocean. These hydrocarbons are toxic to aquatic organisms that reside in the sea.
These rocks are also returned into the ocean which often contain barium ions from the traces of lubricant that remain. These barium ions are toxic as they interfere with enzyme activities which can result in death of living organisms.
Furthermore, there are potassium ions in the machine lubricants in extracting the hydrocarbons (e.g. petroleum & natural gas) where high levels of potassium can result in uncontrollable algae growth leading to a state of eutrophication. This essentially allows algae to grow on the water surface which blocks the sunlight reaching to the plants beneath the water as well as oxygen gas that is dissolved in the water. This will result in the death of plants which decreases the oxygen availability in the water for aquatic animals like fish as well as bacteria that further uses more oxygen to decomposes dead matter of demised plants. Overall, it destroys the aquatic ecosystem turning a habitable environment into a toxic one for the original species.
Other than eutrophication, an increase in ion concentration (e.g. K+ ions) in the surrounding water can disrupt the osmotic balance in living organisms, resulting in the deformations in aquatic fauna or flora.
Another environmental implication would involve the noise pollution through the sending of sound waves to detect potential hydrocarbon deposits for drilling. This would disturb local aquatic organisms, such as whales, as well as any humans that near the area. The sound waves can disorientate whales which can result in large scale whale stranding on beaches. This results in their death through multiple means on is due to dehydration.
To obtain hydrocarbons for use, it must be transported from the sea to land to oil refinery so that petroleum can be split into their components. The mode of transportation is through the use of ships. Oil spillages due to various accidents such as collisions with rocks, other ships, etc have resulted in environmental damages similar to those which we have mentioned already. These hydrocarbons can be washed ashore which can pollute beaches whereby humans have contact with.
Hydrocarbons that enter the human body could cause severe respiratory irritation. The long term effects of hydrocarbon exposure is also currently not fully understood.
Economic Implications
Through potential implications such as leaking toxic hydrocarbons, potassium ions and eutrophication as we have discussed earlier as an environmental implication, they could all result in the death and reduction in biodiversity of aquatic organisms. It is important to preserve biodiversity for many countries as aquatic organisms serves a major source of economic revenue.
For example, aquatic organisms sold from Philippines accounts for over $550 billion USD dollars annually. The reduction of Philippines’ aquatic organisms would result in a major hit to the country’s economy and have many social implications such as poverty (which is relevant for the following section – Sociocultural Implications of obtaining hydrocarbons).
Philippines recognises the importance of preserving biodiversity of its aquatic organisms as it is first south eastern asian country to regulate the risk of biotechnology as the technology could reduce the country’s rich aquatic organisms biodiversity.
Sociocultural Implications
Workers involved in the drilling process to obtain hydrocarbons will be exposed to drilled rocks that are covered in toxic hydrocarbon lubricants, as well as the lubricants in the machinery itself, which can be accidentally or voluntarily inhaled as oil mists in the air.
We have already mentioned how the leaking of toxic hydrocarbons, potassium ions and eutrophication during the extraction of hydrocarbons can result in a decline in aquatic organism’s biodiversity. This has many implications in removing or limiting the diverse range of food in which humans can enjoy in our everyday lives. Also, as the supply of aquatic organisms decreases due to hydrocarbon pollution, the price of seafood would increase which means that it would be less affordable for the global population in general.
Furthermore, the presence of toxic hydrocarbons would result in our everyday potable (drinkable) water derived from the sea being toxic. The treatment of water would thus be more thorough which would incur additional cost to consumers.