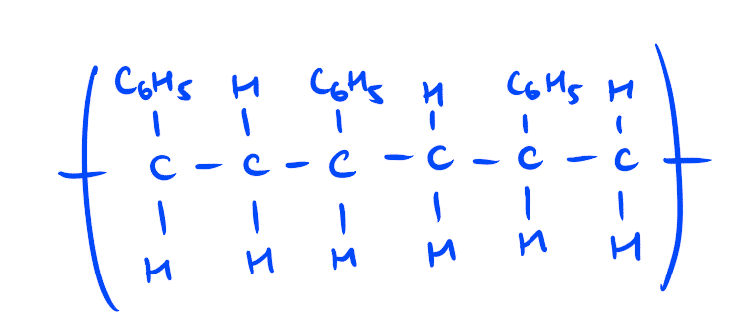HSC Chemistry Syllabus Notes -
Module 7 / Inquiry Question 6
Overview of Week 13 Inquiry Question – What are the properties and uses of polymers?
Learning Objective #1 – Model and compare the structure, properties and uses of addition polymers of ethylene and related monomers, for example:
Polyethylene (PE)
Poly(vinyl chloride) (PVC)
Polystyrene (PS)
Polytetrafluoroethylene (PTFE)
Learning Objective #2 – Model and compare the structure, properties and uses of condensation polymers, for example:
Nylon
Polyesters
NEW HSC Chemistry Syllabus Video – Polymers
Week 13 Homework Questions (Essential for Band 5!)
Week 13 Curveball Questions (Moving from Band 5 to Band 6!)
Week 13 Extension Questions
Solutions to Week 13 Questions
Learning Objective #1 - Model and compare the structure, properties and uses of addition polymers of ethylene and related monomers, for example:
- Polyethylene (PE)
- Poly(vinyl chloride) (PVC)
- Polystyrene (PS)
- Polytetrafluoroethylene (PTFE)
When dealing with this type of question where you are expected to compare the structure, properties and uses of a substance (e.g. a polymer), we recommend you to use the following guideline:
You should always relate how the chemical structure of the substance governs or determines the physical or chemical property of the substance.
You should always relate one chemical structure to one physical or chemical property.
Subsequently, you should relate how the physical or chemical property of the substance (due to its chemical structure) is the reason why it is used for a specific application.
You should follow this process as it follows the marking criteria for HSC Chemistry Questions.
What are Addition Polymers?
Addition polymerisation is a polymerisation reaction that involves the breaking of saturated monomer units (e.g. double bond between atoms) to combine repeating monomer units together without the loss of atoms in resulting polymer, i.e. no elimination of a small molecule.
That is, addition polymers are formed when monomers combining together without the loss of a small molecule as a by-product.
What to look out for:
Double bond present in monomer that is broken to bond with another monomer unit. Usually the double bond that is broken is between carbon atoms.
The monomer can be same or different (98% of the time the monomers are same for HSC Chemistry for addition polymerisation).
The polymer no longer have the double bond that were present in its monomer units.
No small molecule is eliminated as a by-product.
Low Density Polyethylene (LDPE) and High Density Polyethylene (HDPE)
Structure of low density polyethylene
Structure of high density polyethylene (3 monomer units)
Draw the HDPE structure if HSC Chemistry exams request the polymer structure of “polyethylene” in general.
As the manufacturing process of low-density polyethylene (LDPE) does not use a catalyst, it has a high degree of chain branching which results in its carbon chains not being able to be packed closely together. Chain branching is the process where an alkyl group replaces a hydrogen atom that is attached to the carbon chain, resulting in a non-linear carbon chain.
Comparatively, as the manufacturing process of high-density (HDPE) uses a catalyst, monomer chains can only propagate in one direction rather than randomly in LDPE. Therefore, this results there is little to no chain branching in HDPE. This means that carbon chains in HDPE are able to pack more closely together than in LDPE.
Hence, due to the difference in chain branching, there is stronger dispersion force between the carbon chains in HDPE than in LDPE which results in HDPE having a higher melting and boiling point. For the same reason, due to chain branching, it makes HDPE more dense, tough and inflexible whilst LDPE less dense, soft and more flexible.
Due to the carbon chains being closely packed together in a more ordered fashion in HDPE compared to LDPE, there is more crystalline regions in HDPE. Due to the HDPE’s higher number of crystalline regions, it is able to more effectively scatter light which results in HDPE being translucent or semi-transparent. In the case of LDPE, it is transparent as it has an average of less than twice the number of crystalline regions compared to HDPE.
Both LDPE and HDPE are non-polar as they have a linear structure do not contain polar bonds but rather just C-H bonds. Due to this, both polymers are insoluble in water, or water resistant, as they cannot form hydrogen bonds when they come into contact with water.
As the high energy C-C and C-H bonds in LDPE and HDPE are very strong, they result in the polymers being resistant to chemical attacks.
Due to LDPE being transparent, light, flexible, chemically resistant, and water insoluble, it is used in water bottles and cling wraps to lightly package water and food respectively where the packaged item can be seen easily. It is for the same properties that justifies the use of LDPE in plastic shopping bags so that purchased items can be carried as lightly and easily as possible.
Comparatively, due to the rigid and inflexible nature of HDPE, the polymer is used to make plastic buckets and freezer bags to carry heavier substances. Additionally, an ultraviolet absorber is usually incorporated into the structure of HDPE during its manufacturing process so that the polymer can be used to make garbage bins and petrol tanks. This is because the resulting HDPE will be rigid, inflexible, chemically resistant and will also not be decomposed by ultraviolet light.
Poly(vinyl chloride) - PVC
The following diagram shows the structure of poly(vinyl chloride) with three monomer units.
Compared to LDPE and HDPE, the presence of a chlorine atom attached to each carbon in each PVC carbon chain means that there is greater polymer chain stiffness in PVC. This is because chlorine atoms in PVC are larger in diameter than a hydrogen atom in polyethylene. Due to this, pure PVC is more rigid than HDPE and LDPE. Additionally, the C-Cl bonds in PVC’s carbon chains allow PVC to form dipole-dipole forces between polymers chains, resulting in pure PVC having a higher melting & boiling point than HDPE and LDPE. That being said, a plasticiser additive can be incorporated in PVC’s structure between carbon chains during the polymer’s manufacturing process to increase the distance between carbon chains. This effectively weakens the dispersion force between carbon chains. For such reason, this soft PVC is more flexible than HDPE.
As the C-Cl bond in PVC has lower bond energy than the C-H bond in polyethylene, pure PVC is prone to being decomposed when exposed to ultraviolet radiation. For such reason, an ultraviolet inhibitor such as TiO2 is incorporated in PVC’s manufacturing process such that additive can absorb the UV radiation without decomposing when exposed to daylight.
In terms of uses, the PVC polymer after adding UV absorber and plasticiser additive be used to make gardening hoses. This is because the polymer is water insoluble, flexible and will not decompose when exposed to ultraviolet radiation in sunlight.
Although the C-Cl bonds in poly(vinyl chloride) are polar, the polymer’s linear structure and high overall number of non-polar C-C and C-H bonds considerably weakens the strength of the dipole-dipole bond that PVC can form with water. Due to this chemical structure, PVC is insoluble in water.
To improve PVC’s poor thermal stability attributed to its structure impurities, a heat stabiliser molecule is incorporated its structure to increase the melting point of PVC. Due to this flexible and water-resistant PVC is able to be used transporting hot water through hot water pipes.
As there are no unshared lone pairs of electrons in PVC, it is a poor conductor of electricity. Coupling this property with PVC’s high flexibility and good heat stability after adding plasticiser and ultraviolet inhibitor, PVC is therefore suitable to be used as electrical conduits.
Crystal Polystyrene vs Expanded Polystyrene
The following diagram shows the structure of polystyrene with 3 monomer units
As benzene molecule is larger in diameter than a hydrogen atom, the presence of a phenyl ring attached to each carbon in polystyrene’s polymer chains results in greater polymer chain stiffening than in polyethylene and PVC. Due to chain stiffening and the presence of impurities resulting in crossing-linking bonds formed between polymer chains, polystyrene is more rigid and brittle than polyethylene and PVC.
Due to crystal polystyrene being more rigid than polyethylene and PVC, it is suitable to be used in car battery cases, hard plastic furniture, and tool handles.
As crystal polystyrene has very little crystalline regions in its chemical structure, it is transparent. Crystal
polystyrene can be moulded into a particular shape to meet their use. During the moulding process, gas such as carbon dioxide is injected into molten polystyrene which is subsequently allowed to cool. Upon cooling, the
polystyrene will adopt the structure of the mould shape whilst retaining its rigidness. Due to the presence of trapped gas in the polystyrene that are not allowed to circulate readily, the heat transfer from the polystyrene to the environment is reduced significantly. Crystal polystyrene is also chemical resistant to redox reactions due to the presence of the phenyl group. Therefore, due to polystyrene’s ability to be moulded, high transparency, good heat insulator and resistant to chemical redox reactions, it is used in food containers, plastic eating utensils, transparent drinking cups and heat-pressed food packaging.
Similar to crystal polystyrene, expanded polystyrene is able to be moulded by injecting gas such carbon dioxide into molten polystyrene which is subsequently allowed to cool. Due to the presence of trapped gas in the polystyrene that are not allowed to circulate readily, the heat transfer from the polystyrene to the environment and sound waves travelling through the polymer are reduced significantly. Moreover, due to expanded polystyrene is made of gas, most of its weight are distributed over a larger area which allows it to be light. Furthermore, as the expanded polystyrene is mostly air, it has high compressibility which allows it to act as an excellent shock absorbent. For such reasons, expanded polystyrene is used as protective packaging material.
Polytetrafluoroethylene (PFTE)
The following diagram, diagram 5, shows the structure of polytetrafluoroethylene with 3 monomer units.
Due to minimum chain branching, polytetrafluoroethylene (PTFE) have a high number of crystalline regions which allows it to scatter light effectively. This results in PTFE being translucent to opaque polymer in appearance. Furthermore, with minimal chain branching, the polymer chains are able to pack very closely together to form strong intermolecular (dispersion and dipole-dipole) forces. Due to this, the polymer has a high melting and boiling point. Due to the presence of three lone pairs of electron for both fluorine atoms attached to each carbon atom, the repulsion between fluorine atoms is high and hinders free CF2 bond rotation. That being said, the absence of large bulky groups such as chlorine and phenyl ring in PVC and polystyrene respectively, allows PTFE to not be as rigid.
The high bond energy in the strong carbon-fluorine bond in PTFE can be attributed to fluorine’s high electronegativity. This means that most of the electrons will spend more time around the fluorine atom so that it is difficult to polarise the C-F bond and large energy is required to substitute the fluorine atom from the polymer. This allows PTFE to be chemically unreactive and resistant to chemical attacks. Moreover, an appreciable amount of hydrogen bonding involving PTFE with another substance such as water is also difficult. This is because C-F does not form hydrogen bond with hydrogen in water due to the F- electrons ‘orbit’ close to fluorine atoms’ nuclei. Due to this, PTFE is water-repellent or insoluble in water.
Therefore, due to the strong C-F bond and inability for the polymer to hydrogen bond, PTFE is also able to have useful non-stick and low friction properties.
Due to its high melting and boiling point, chemically unreactive, non-stick, low friction and water-insoluble properties, polytetrafluoroethylene is used as the surface for non-stick cooking pans. When used in restaurants, the polymer allows faster and easier cleaning so customers’ orders can be made faster.
PTFE is also in pipelines to transfer hot chemicals as it is chemically unreactive and have a high melting and boiling point. This will reduce the frequency in which pipelines will have to be replaced which effectively minimises downtime in business operations.
Due to the low friction property of the polymer and high melting point, it is used as a coating for machine parts to not only reduce the wear and tear due to friction and high temperature but to also increase the operational efficiency of machines.
Learning Objective #2 - Model and compare the structure, properties and uses of condensation polymers, for example:
-Nylon
- Polyesters
Condensation Polymer
Condensation polymers are formed via condensation polymerisation.
Condensation Polymerisation: A polymerisation reaction to that involves two (different or identical) monomers combine and a functional group from each monomer react to eliminate a small molecule as a by-product.
That is, condensation polymers are formed when monomers combine together with the loss of a small molecule as a by-product.
The small molecule can be water, alcohol, hydrogen chloride, etc.
For 98% of the time, the small molecule would be water for HSC Chemistry.
What to look out for:
Monomer with a functional group on each end (can be identical functional group or different)
The monomer can be identical or different.
A small molecule is eliminated as a by-product when two monomer units join together.
The monomer can have a double bond. However, the double bond is still present in the polymer.
Nylon
Nylon is an example of a type of polymer called polyamides which belongs to the class of polymer called condensation polymer.
You could probably guess that condensation polymers are formed via condensation polymerisation.
There are two common variants of nylon which are Nylon 6 and Nylon 6,6.
That being said, we will NOT be exploring Nylon 6 as it is not a polymer formed via condensation polymerisation.
So, we will move on to examine Nylon 6,6.
The, Nylon 6,6, is polyamide polymer can be formed using two different types of monomers. One of the monomer units comprises of two COOH groups and another monomer unit with two NH2 functional groups.
The nylon 6,6 polymer is synthesised through reacting Hexane-1,4-dioic acid and 1,6-diaminohexane via a condensation reaction in the presence of high temperature (500K+) and high pressure.
When two monomer unit joins to form the polymer, a water molecule is eliminated and, thus, produced as a by-product (where the polymer is ‘main’ product)
That is, (2n-1) water molecules are formed as a by-product where n = the number of monomer unit.
NOTE: The word ‘hexane’ in the monomer name is named based on the number of carbon atoms that is present between the functional groups in each monomer.
This therefore means that for each one monomer unit of the polymer,
Nylon 6,6’s polymer chains are closely packed together, resulting in an high degree of intermolecular forces (e.g. strong hydrogen bonds) between polymer chains. This results in the polymer’s high rigidness, durability elasticity as well as high melting & boiling point. It has a melting point of 270 degrees celsius.
Due to the high crystallinity region between the polymer chains when polymer is drawn into fibres (matted), it allows the polymer to high tenacity.
NOTE: As Nylon is a thermoplastic, it can be melted to break the intermolecular forces and the polymer can be reshaped and allowed to cool. The polymer does not decompose as covalent bonds are not broken. This is why Nylons can be woven into fibres. Also, as the polymer can be remoulded into a different shape, it makes them easy to recycle to create a new product.
NOTE: Thermosetting polymers are different to thermoplastic polymers in that the covalent bonds are broken instead of the intermolecular forces due to the high strength of the intermolecular forces. As a result, they decompose or burn when heated.
Coupled with the strong and high extent of intermolecular forces, the extent of polymerisation in nylon is very high which means that there many monomer units in each polymer chain (i.e. the average molecular weight of polymer chains are very high). This allows the polymer to have high tensile strength (pulled left and right across cross-section) as they exhibit less stress to strain.
Due to these these reasons, Nylon 6,6 is a polymer used to manufacture ropes and synthetic fibres.
That being said, fibres made from Nylon 6,6 are weak when pulled from above and below (longitudinally) . For such the reasons, polyesters are used as to make synthetic fibres too.
Furthermore, because the polymer is rigid (reason explained earlier), solid (i.e. not woven fibre) Nylon 6,6 can be used in place of metal bearings or wheels in pulleys, machine screws, etc thus lowering the cost in the event of replacement.
Due to the close packing of polymer chains, alongside the presence small, even spaced pores, it makes nylon-6,6 difficult to dye. That being said, once the polymer is dyed, the incorporated dye molecules is less susceptible to breaking from photons (energy) from sunlight and therefore less susceptible to fading. Also for such reason, it makes Nylon 6,6 having stain resistance properties. These properties, coupled with the high tensile strength, elasticity and tenacity properties, makes it desirable for Nylon to be used in the manufacturing of coloured-woman stockings when it is matted.
NOTE: Nylon-6,6 only has moderate resistance to U.V radiation.
Polyesters
Dacron (Terylene) and Kodel are examples of another type of polymer called polyester which belongs to the class of polymer called condensation polymer.
There are two monomer situations in which a polyester polymer can be produced:
Situation #1: Two different types of monomers are required. One of the monomer unit would need to have two COOH groups, i.e. two carboxylic acid groups. The other monomer unit would need to have two OH groups, two hydroxyl groups.
Situation #2: Only one type (identical) monomer is required. This monomer will have one carboxylic acid functional group on end and an hydroxyl functional group on the another end.
In either case, an ester bond is formed where OH group reacts with a COOH group to form the COO bond (one C=O and one C-O bond) and a water molecule is released as a by-product.
Dacron (or Terylene) is also known as PET, i.e. poly(ethylene terephthalate) which produced via the condensation polymerisation reaction between dimethyl terephthalate and ethylene glycol.
It involves heating the mixture of the two monomers at 420-460K with a zinc acetate-antimony trioxide catalyst.
For every one-monomer-unit Dacron polyester that is produced, two methanol molecules are produced as a by-product of the condensation reaction.
The aromatic ring in PET has high stability as it is conjugated which provides resonance, allowing the delocalisation of electrons across multiple atoms in the ring. This makes PET unreactive or chemically inert.
On top of that PET is a thermoplastic as heating the polymer will break the intermolecular forces between polymer chains but will not break the covalent bonds within polymer chains. As a result, the polymer will not decompose and can be remoulded into a different shape that is suitable for packaging or recycling.
Due to PET being chemically inert and can be moulded into different shapes, it can be used to package food as no reaction between the PET and chemicals (acids/bases) in the food would occur. Also, it can be used as bottles for carbonated soft drinks.
NOTE: Due to the presence of the aromatic ring in PET, it makes the polymer chains rigid which is property that justifies the use in food packaging, however, it also makes the polymer not biodegradable. The rigidness of PET due to the aromatic ring prevents enzymes to bind the polymer to its active site and decompose the polymer.