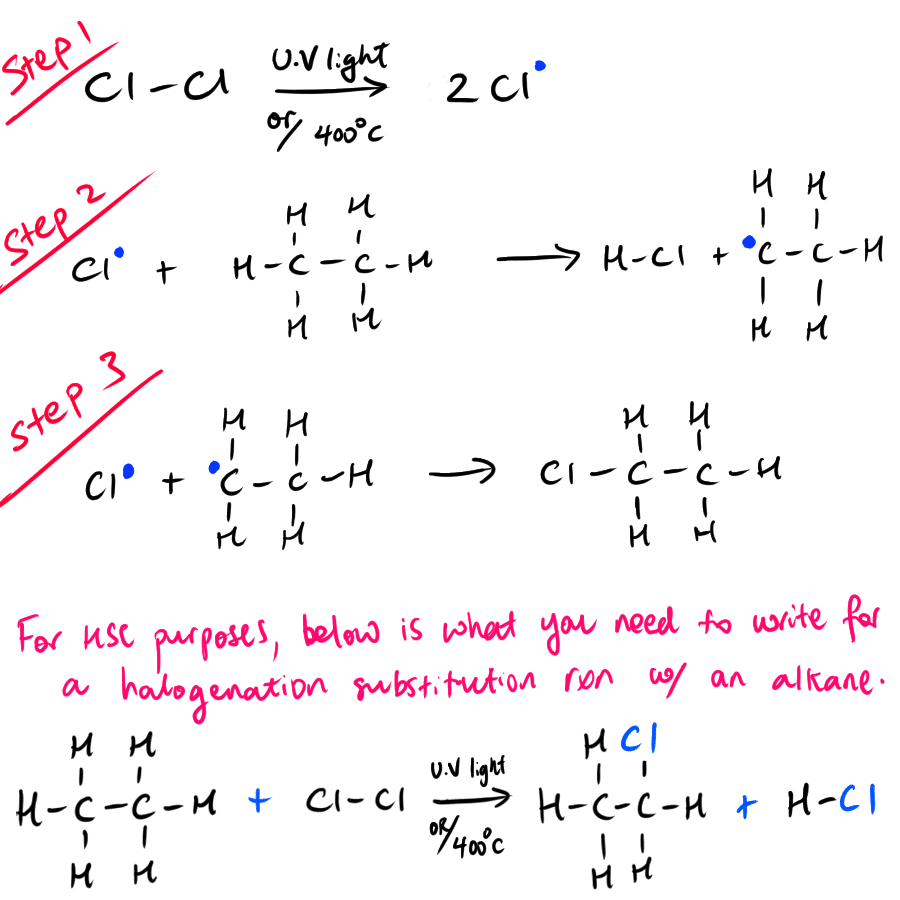HSC Chemistry Syllabus Notes
Module 7 / Inquiry Question 3
Overview of Week 10 Inquiry Question – What are the products of reactions of hydrocarbons and how do they react?
Learning Objective #1 – Investigate, write equations and construct models to represent the reactions of unsaturated hydrocarbons when added to a range of chemicals, including but not limited to:
Hydrogen (H2)
Halogens (X2)
Hydrogen Halides (HX)
Water (H2O)
Learning Objective #2 – Investigate, write equations and construct models to present the reactions of saturated hydrocarbons when substituted with halogens.
NEW HSC Syllabus Video – Products of Reactions Involving Hydrocarbons
Week 10 Homework Questions
Week 10 Curveball Questions
Week 10 Extension Questions
Solutions to Week 10 Questions
Overview of Week 10 Inquiry Question
Welcome back to Week 10 of your HSC Chemistry Syllabus Notes!
This week’s content is fairly straightforward.
In the previous two weeks, we have explored with a detailed scope into saturated and unsaturated hydrocarbons in terms of their structure, physical properties and chemical properties.
We also did comparisons between homologues within a homologous series as well as between corresponding homologues in different homologous series.
Now, in this week’s notes, we are going to explore the different kinds of reactions that saturated and unsaturated hydrocarbons can undergo.
In the this week’s youtube video, we will explore other different types of reactions in which saturated and unsaturated hydrocarbons can undergo. Without further ado, let’s get started!
Learning Objective #1 - Write equations and construct models to represent the reactions of unsaturated hydrocarbons when added to a range of chemicals
Hydrogenation: Adding hydrogen (H2) to unsaturated hydrocarbons
Hydrogenation is an example of an addition reaction whereby, in our case, hydrogen gas reacts with unsaturated hydrocarbons such as alkenes and alkynes to form one combined product.
In hydrogenation specifically, the hydrogen reacts with and breaks the C=C bond such that one hydrogen atom is added (i.e. covalently bonded) to each carbon atom whereby the alkene is converted into an alkane product.
That is, the carbon that was originally unsaturated became saturated after hydrogenation.
NOTE: In the exam I would recommend writing H2 as a reactant.
i.e.. C2H5(g) + H2(g) -> C2H6(g)
Halogenation: Adding Halogens (X2) to unsaturated hydrocarbons
Another type of addition reaction is halogenation whereby diatomic halogen molecules reacts with the unsaturated carbon bond, e.g. C=C bond, resulting in one halogen atom being added to each carbon atom.
The resulting product is a haloalkane (alkane containing halogen atom(s))
This effectively converts the unsaturated carbon in an alkene into an saturated carbons in an alkane.
NOTE: The halogen molecule (X2) that reacts with the unsaturated hydrocarbon (e.g. ethylene in this case) could be Cl2, F2, Br2, etc.
Hydrohalogenation: Adding Hydrogen Halides (HX) to unsaturated hydrocarbons
Hydrohalogenation is the third type of addition reaction in which a hydrogen halide (HX) molecule breaks the C=C bond so that a hydrogen is added to one carbon atom and a halogen atom is added to the other carbon.
The result is a haloalkane being produced.
In HSC Chemistry, we do not need to need to understand which carbon atom the hydrogen is added to and which carbon atom the halogen is added to. It is because it is outside the HSC Chemistry Syllabus.
However, we will explore the Markovnikov’s rule in this week’s Youtube Video so that we can create a more accurate model for haloalkane that is produced via hydrohalogenation reaction.
Again, you can simply add to any of the two unsaturated carbon as you wish for HSC Chemistry purpose if you decide to not learn the Markovikov rule as it’s not part of HSC Chemistry syllabus. This is because Markovnikov’s Rule is outside the scope of HSC Chemistry.
Hydrohalogenation of unsaturated hydrocarbon (without Markovnikov's Rule)
NOTE: The hydrogen halide (HX) molecule that reacts with the unsaturated hydrocarbon does not necessarily have to be HBr. It can be other halogens such as HF, HCl, etc.
Hydration: Adding Water (H2O) to unsaturated hydrocarbons
The final addition reaction is hydration. In hydration, there is a hydroxyl group (-OH) that is added to the unsaturated carbon atom (i.e. part of the C=C bond) and a hydrogen atom is added the other carbon atom which was part of the original C=C bond.
The resulting product is a saturated alcohol.
Now it's alkynes' turn!
We have explored one type of unsaturated hydrocarbon which was alkenes.
The second type of unsaturated hydrocarbons is alkyne. So let’s explore how alkynes can undergo additional reaction.
NOTE: Alkynes can double the number of chemical reaction with halogens compared to alkenes. This is because alkynes have triple carbon bonds while alkenes have double carbon bonds.
We will only explore halogenation and hydrohalogenation addition reactions for alkynes as the other reactions we have explored for alkenes will follow the same principle.
That is, alkynes will have double the number of addition chemical reactions that alkenes can perform.
Halogenation of unsaturated hydrocarbons - Alkynes
Hydrohalogenation of unsaturated hydrocarbons - Alkynes
Learning Objective #2 - Write equations and construct models to represent the reactions of saturated hydrocarbons when substituted with halogens.
By now, you should know that saturated hydrocarbons refers to alkanes molecules where there are only single covalent bonds.
Due to the high bond energy of the C-C and C-H bonds coupled with the fact that unbranched alkanes does not have a functional group so they only exhibit dispersion forces as they are non-polar.
Henceforth, they are commonly considered chemically unreactive as they do not readily undergo many chemical reactions.
As alkanes only contain single bonds, it is not possible for them to undergo addition reactions as carbon can only have four bonds.
That being said, it is possible for saturated hydrocarbons (alkanes) to undergo substitution reactions under the presence of high temperatures or ultraviolet radiation to break the C-H bond.
Also, they can also undergo combustion reactions (i.e. redox reaction) involving the breaking C-C and C-H bonds in high temperature and pressure such as in car engines.
Substitution Reaction involving halogens
Using heat energy (200 to 400 degrees celsius) or ultraviolet radiation, it is possible to break the covalent bonds (X-X) between halogen atoms in a halogen molecule. This effectively creates a halogen free radical that can react readily with a hydrogen atom in the saturated hydrocarbon.
This gives two products being a radical hydrocarbon molecule that with a missing hydrogen atom and a hydrogen halide.
The radical hydrocarbon molecule can react with a free halogen radical to form an alkyl halide (R-CH2-X) (also known as halogenated alkane or haloalkane).
The effect is that a hydrogen atom in the original saturated hydrocarbon is substituted with a halogen atom.
This process is summarised in the diagram below.
NOTE: The alkyl halide product can further undergo more substitution reactions to where progressively more hydrogen atoms are being substituted with halogen atoms.
Now we have talked about the halogenation substitution reaction for saturated hydrocarbons (alkanes). What about hydrohalogenation?
For hydrohalogenation of alkanes, the halogen is added to the saturated carbon atom in the hydrocarbon so that a hydrogen atom is substituted out of the original hydrocarbon. This is exactly the same as the case of hydrohalogenation.
Therefore, the product of of hydrhalogenation of a saturated hydrocarbon is an alkyl halide like in above case of halogenation.
However, hydrogen gas is produced rather than a HX molecule. This is because the substituted hydrogen atom from the original alkane bonds with the hydrogen from the original ‘HX’ reagent used in the hydrohalogenation process, forming a hydrogen gas molecule.